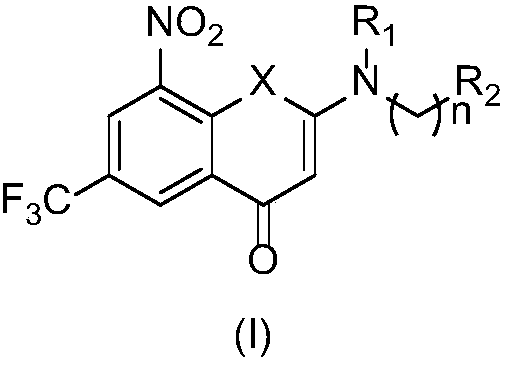
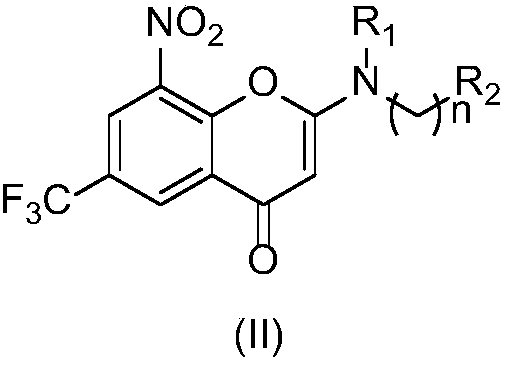
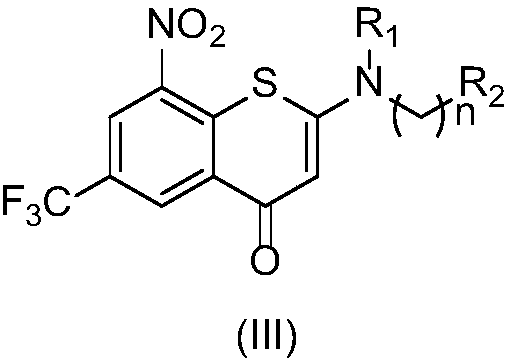
2-substituted amino-5-trifluoromethyl-8-nitrobenzo(thio-)pyran-4-one compound, preparation method therefor and use of 2-substituted amino-5-trifluoromethyl-8-nitrobenzo(thio-)pyran-4-one compound
A technology of compounds and substituents, applied in the field of medicine, can solve problems such as adverse reactions of patients, difficulty in regular drug use, threats to tuberculosis prevention and control, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0078] The present invention can be described in detail by the following examples, but it does not imply any adverse limitation on the present invention. The present invention has been described in detail herein, and its specific embodiments are also disclosed. For those skilled in the art, it is necessary to make various changes and improvements to the specific embodiments of the present invention without departing from the spirit and scope of the present invention. Obvious.
[0079] For all of the following examples, standard manipulations and purification methods known to those skilled in the art can be used. All temperatures are in °C (degrees Celsius) unless otherwise indicated. The structures of the compounds were determined by nuclear magnetic resonance spectroscopy (NMR).
[0080] Preparation of the Example section
[0081] The structure of the compound was obtained by H NMR spectroscopy ( 1 H NMR) to determine. Proton and Carbon NMR shifts (δ) are given in part...
Embodiment 1
[0096]
[0097] 2-((Phenyl)amino)-6-trifluoromethyl-8-nitro-chromen-4-one
[0098] route:
[0099]
[0100] Experimental steps:
[0101] Preparation of the first step 3-(2-chloro-3-nitro-5-(trifluoromethyl)phenyl)-3-oxo-N-phenylpropanamide B-1
[0102] In a 25mL reaction bottle, the compound 3-(2-chloro-3-nitro-5-(trifluoromethyl)phenyl)-3-oxopropionic acid A (311mg, 1.0mmol), DCC (201mg, 1.0 mmol) was dissolved in dry dichloromethane (5 mL), protected by Ar, aniline (93 mg, 1.0 mmol) was added, and stirred at room temperature for 12 hours. The insoluble solids were filtered off, concentrated, and separated by silica gel (200-300 mesh) column chromatography, using ethyl acetate-petroleum ether (V:V=5-15:100) mixture as the eluent. Intermediate B-1 was obtained, 218 mg of light yellow solid, yield 56.5%.
[0103] 1 H NMR (400MHz, CDCl 3 )δ: 14.19(s, 1H), 8.05(d, J=8.4Hz, 2H), 7.54(d, J=7.6Hz, 2H), 7.38(t, J=7.6Hz, 2H), 7.18(t, J=7.6Hz,1H),7.11(brs,1H),5.57(s,1H). ...
Embodiment 2
[0108]
[0109] 2-(((2,3-dihydrobenzo[b][1,4]dioxin-6-yl)methyl)(methyl)amino)-6-trifluoromethyl-8 -Nitro-benzopyran-4-one
[0110] route:
[0111]
[0112] Experimental steps:
[0113] The first step 3-(2-chloro-3-nitro-5-trifluoromethylphenyl)-N-((2,3-dihydrobenzo[b][1,4]dioxane Preparation of En-6-yl)methyl)-N-methyl-3-oxopropionamide B-2
[0114] Using 1-(2,3-dihydrobenzo[b][1,4]dioxin-6-yl)-N-methylmethylamine (179mg, 1.0mmol) as raw material, using the example The first step in 1 was similarly operated to obtain intermediate B-2, 285 mg of light yellow solid, with a yield of 60.3%.
[0115] 1 H NMR (400MHz, CDCl 3 )δ:15.57(m,1H),8.02(m,2H),6.86(m,1H),6.81(m,1H),6.70(m,1H),5.71(m,1H),4.50(m,2H ),4.26(m,4H),3.02(m,3H).
[0116] The second step 2-(((2,3-dihydrobenzo[b][1,4]dioxin-6-yl)methyl)(methyl)amino)-6-trifluoromethane Preparation of yl-8-nitro-benzopyran-4-one II-2 (compound 2)
[0117] With 3-(2-chloro-3-nitro-5-trifluoromethylphenyl)-N-((2,3-dihydro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com