Application of isoniazide as histone deacetylase inhibitors
A deacetylase and histone technology, used in anti-inflammatory agents, drug combinations, non-central analgesics, etc., can solve the problems of cell wall rupture and unexplained mechanism of action, and achieve the promotion of histone acetylation, inhibition and prevention. Effects of organ transplant rejection, suppression and prevention of autoimmune diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
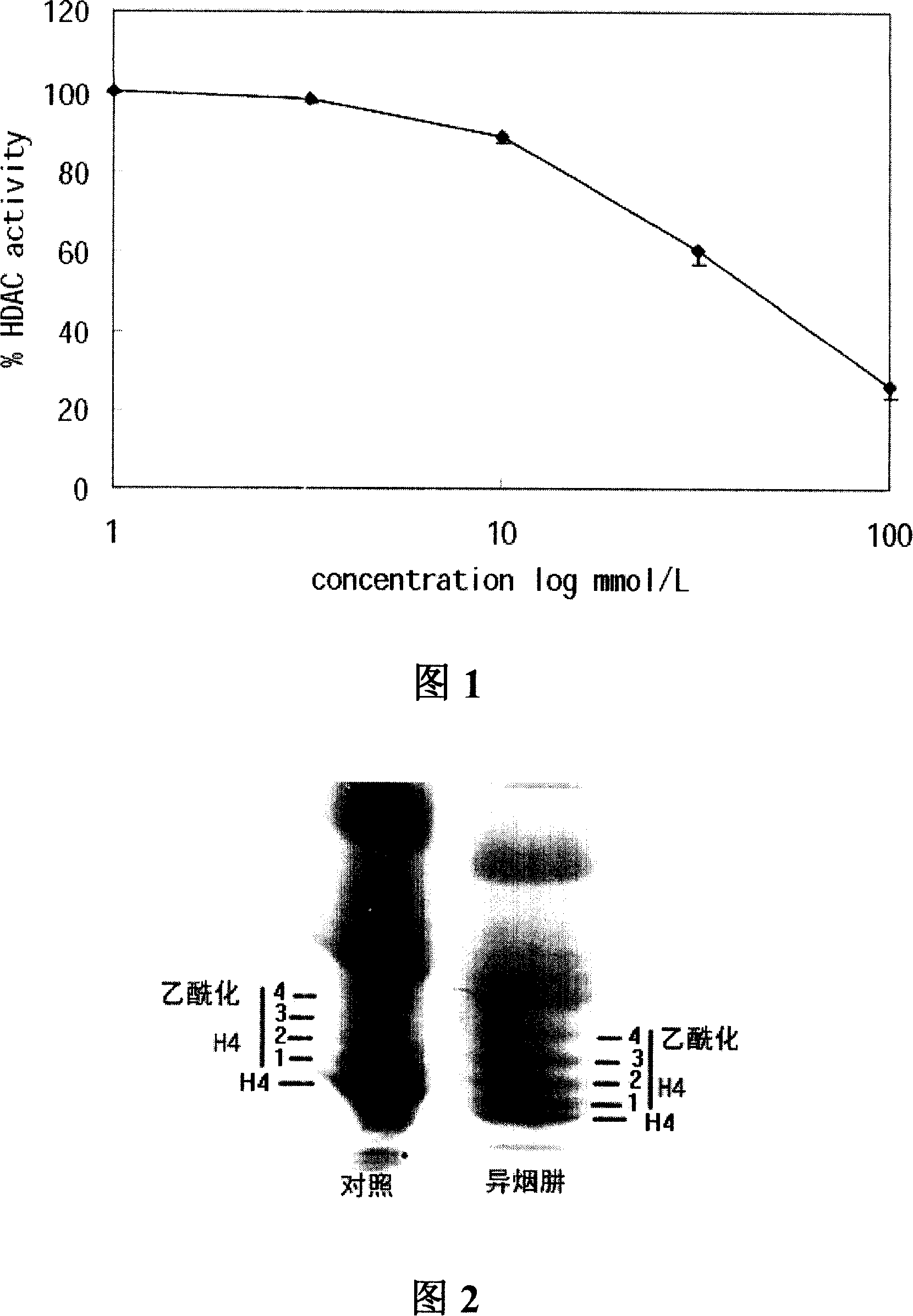
[0040] Example 1: Inhibition of isoniazid on histone deacetylase
[0041] The method for the determination of histone deacetylase activity refers to the literature (Heltweg B, Jung M. Amicroplate reader-based nonisotopic histone deacetylase activity assay. Anal Biochem 2002; 302: 175-183) with some changes.
[0042] Preparation of crude preparation of histone deacetylase: take the liver of healthy Kunming mice, cut it into pieces, add PBS, grind and filter on a 100-mesh sieve, and wash the obtained cell suspension twice with PBS after centrifugation, add an equal volume of PBS to the cell pellet , shake and mix well, lyse the cells by freeze-thaw method, centrifuge (4° C., 12000 rpm, 10 min), absorb the supernatant, add glycerol to a final volume of 30%, and a protein concentration of 5 mg / ml. Freeze at -20°C.
[0043] In the 100 μl reaction system with PBS as the buffer solution, the final concentrations of the reaction components were: MAL 20 μmol / L, enzyme crude preparatio...
Embodiment 2
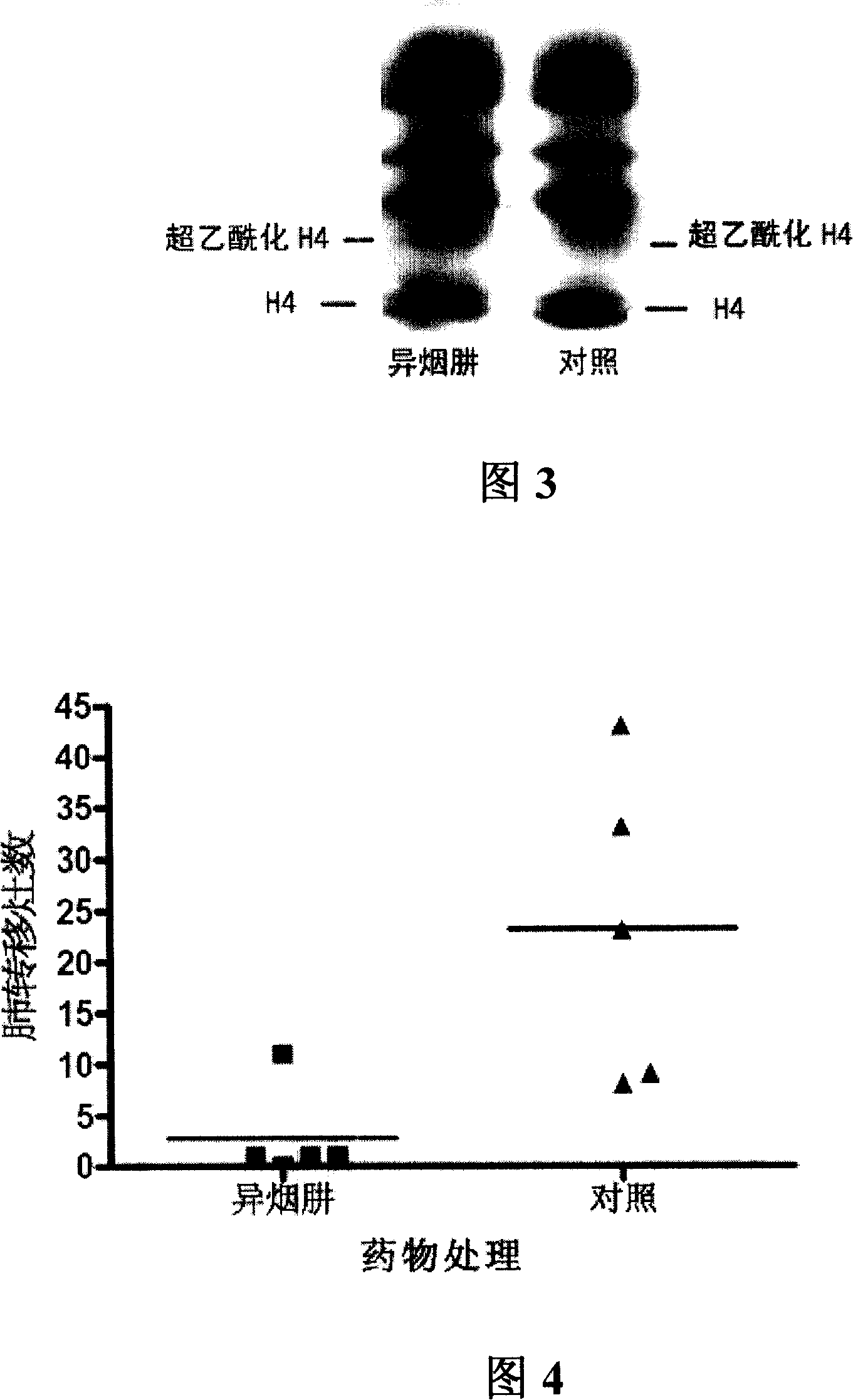
[0046] Example 2: Effect of isoniazid on histone acetylation in cultured cells
[0047] Cell culture and drug treatment: Colon cancer cells HCT-8 cultured in vitro were inoculated in 90mm culture dishes containing 10% calf serum in RPMI1640 complete culture medium, placed at 37°C, 5% CO 2 cultured in an incubator. After the cells grew to the logarithmic growth phase for 24 hours, isoniazid was added to a final concentration of 100 mM, and the culture was continued for 16 hours.
[0048] Histone extraction: Cellular histones were extracted according to the literature (Yoshida, M., Kijima, M., Akita, M., Beppu, T. (1990) J. Biol. Chem. 265, 17174-17179). After the cell incubation, discard the culture medium, scrape the cells with a cell scraper, transfer to an Eppendorf tube, centrifuge at 1500 rpm at 4°C for 5 minutes to collect the cells, wash twice with ice-cold PBS containing 5 mmol / L sodium butyrate, add 100 μl Cell lysate (1xPBS, 5mmol / L sodium butyrate, 0.5% TritonX-100...
Embodiment 3
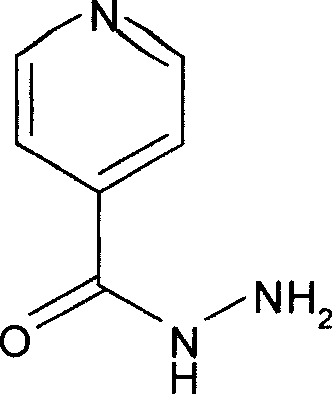
[0051] Example 3: The effect of isoniazid on histone acetylation in whole mouse splenocytes
[0052] Healthy Kunming mice were intraperitoneally injected with isoniazid 100 mg / kg or an equal volume of PBS. Four hours later, they were sacrificed by cervical dislocation. Take the mouse spleen, cut it into pieces, add PBS, grind and filter on a 100-mesh sieve, centrifuge the obtained cell suspension, wash twice with ice-cold PBS containing 5mmol / L sodium butyrate, add 500μl cell lysate (1xPBS, 5mmol / L L sodium butyrate, 0.5% Triton X-100, 5mM PMSF, 0.2% NaN3), lyse the cells at 4°C for 10 minutes. Centrifuge at 2000 rpm at 4°C for 8 minutes to collect cell nuclei, wash twice with ice-cold lysate, add 100 μl of 0.3N HCl, shake and mix well, and place in ice bath for 1 hour. Centrifuge at 12,000 rpm at 4°C for 10 minutes, collect the supernatant, add 1ml of acetone, mix well and place at -20°C overnight. The next day, centrifuge at 12,000 rpm at 4°C for 10 minutes, wash the prec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com