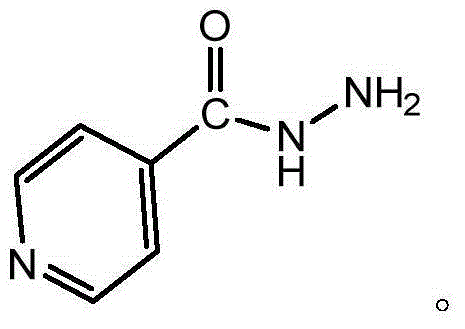
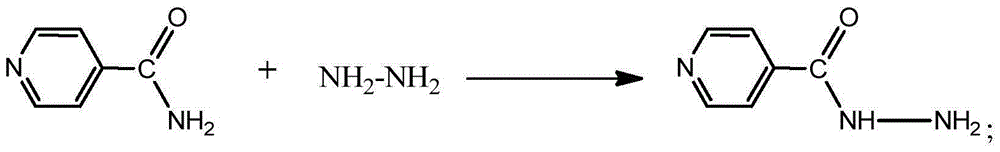Preparation method of isoniazid
A technology of isoniazid and isoniazid, applied in the direction of organic chemistry, can solve the problems of easy generation of impurities, high reaction temperature, high energy consumption, etc., and achieve the effects of reducing waste discharge, reducing costs, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] The preparation of embodiment 1 isoniazid
[0060] Add 200g (1.62mol) of isonicotinic acid, 1200g of ethanol, and 16.4g (0.162mol) of triethylamine into a 2000ml three-necked flask, stir and cool down to 0°C to 5°C, slowly add 212g (1.78mol) of thionyl chloride dropwise, After the dropwise addition, keep the temperature at 0°C to 5°C for 2 hours, then raise the temperature to 20°C to 25°C and keep the temperature for 20 hours. After the reaction is completed, concentrate under reduced pressure to obtain an oil, add 119g (2.43mol) of hydrazine hydrate, and heat up to 70 ℃~75℃, keep warm for 10 hours, add diluent (350g of water), raise the temperature properly, and dissolve to get the aqueous solution of crude isoniazid, add 10g of activated carbon, keep warm at 70℃~75℃ for half an hour, filter, the filtrate is natural Cool for 6 hours, then slowly cool to 0°C, filter, and dry to obtain the finished product of isoniazid, with a purity of 99.94%, a single impurity less tha...
Embodiment 2
[0062] The preparation of embodiment 2 isoniazid
[0063] Add 200g (1.62mol) of isonicotinic acid, 1600g of ethanol, and 16.4g (0.162mol) of triethylamine into a 3000ml three-necked flask, stir and cool down to 5°C to 10°C, slowly add 250g (2.1mol) of thionyl chloride dropwise, After the dropwise addition, keep the temperature at 5°C to 10°C for 2 hours, then raise the temperature to 20°C to 25°C and keep it for 20 hours. After the reaction is completed, concentrate under reduced pressure to obtain an oil, add 143g (2.92mol) of hydrazine hydrate, and heat up to 73 ℃~76℃, keep warm for 10 hours, add diluent (350g of water), raise the temperature properly, and dissolve to get the aqueous solution of crude isoniazid, add 10g of activated carbon, keep warm at 70℃~75℃ for half an hour, filter, the filtrate is natural Cool for 6 hours, then slowly cool to 0°C, filter, and dry to obtain the finished product of isoniazid, with a purity of 99.95%, a single impurity of less than 0.10%, ...
Embodiment 3
[0064] The preparation of embodiment 3 isoniazid
[0065] Add 200g (1.62mol) of isonicotinic acid, 2000g of ethanol, and 16.4g (0.162mol) of triethylamine into a 3000ml three-necked flask, stir and cool down to 5°C-10°C, slowly add 289g (2.43mol) of thionyl chloride dropwise, After the dropwise addition, keep the temperature at 5°C to 10°C for 2 hours, then raise the temperature to 25°C to 30°C and keep it for 20 hours. After the reaction is completed, concentrate under reduced pressure to obtain an oil, add 158g (3.2mol) of hydrazine hydrate, and heat up to 75°C. ℃~80℃, keep warm for 10 hours, add diluent (350g of water), raise the temperature properly, and dissolve to get the aqueous solution of crude isoniazid, add 10g of activated carbon, keep warm at 70℃~75℃ for half an hour, filter, the filtrate is natural Cool for 6 hours, then slowly cool to 0°C, filter, and dry to obtain the finished product of isoniazid, with a purity of 99.93%, a single impurity of less than 0.10%, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com