Chirality 7-(piperazine-substituted pyrazol aldehyde condensation isoniazide) fluoroquinolone carboxylic acid derivative as well as preparation method and application thereof
A technology of fluoroquinolone carboxylic acid and isoniazid, which is applied in organic chemistry, antibacterial drugs, etc., can solve the problems of affecting animal cartilage development, easy drug resistance, and low therapeutic index, so as to increase anti-tuberculosis activity and drug resistance. Strong effect and high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
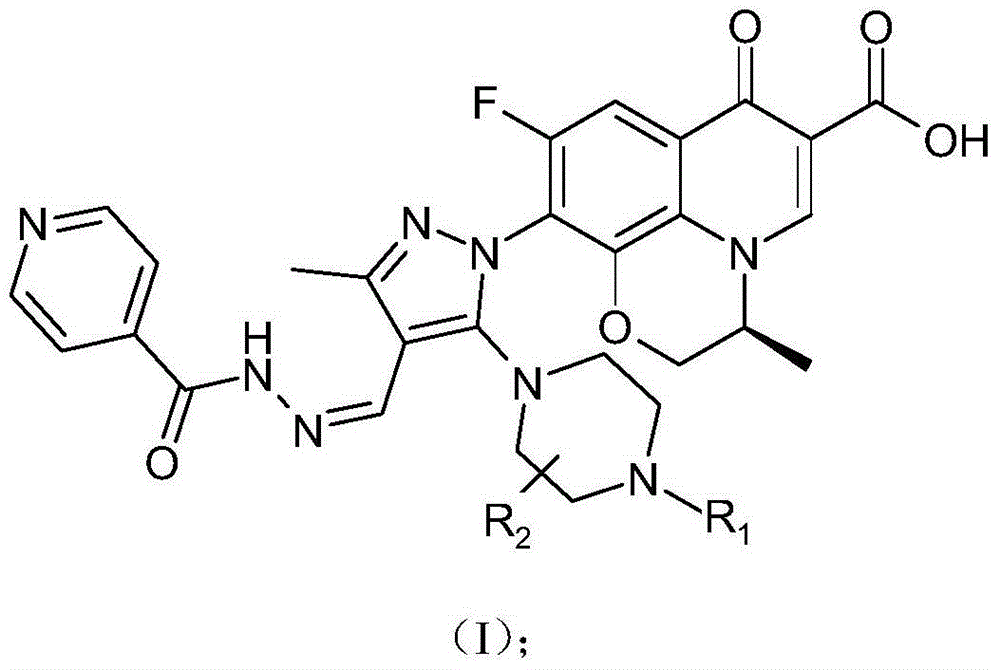
[0050] The chiral 7-(piperazine substituted pyrazole aldehyde isoniazid hydrazone) fluoroquinolone carboxylic acid derivative in this example is (S)-3-methyl-9-fluoro-10-[3-methyl- 4-(Pyridine-4-carbohydrazino)ylidenemethyl-5-piperazin-1-yl-pyrazol-1-yl]-2,3-dihydro-[1,4]oxazino[2 ,3,4-ij] quinoline-7(4H)-one-6-carboxylic acid, its chemical structural formula is:
[0051]
[0052] That is, R in formula (I) 1 is a hydrogen atom, R 2 for a hydrogen atom.
[0053] The preparation method of the chiral 7-(piperazine substituted pyrazole aldehyde isoniazid hydrazone) fluoroquinolone carboxylic acid derivative of this example is: take 1.0 g (2.0 mmol) of (S)-3-methyl- 9-fluoro-10-[3-methyl-5-chloro-4-(pyridine-4-formylhydrazino)ylidenemethyl-pyrazol-1-yl]-2,3-dihydro-[1, 4] Oxazino[2,3,4-ij]quinolin-7(4H)-one-6-carboxylic acid (VI) and 0.34g (4.0mmol) of anhydrous piperazine were added to 20ml of anhydrous acetonitrile The reaction was refluxed for 12 hours and left overnight...
Embodiment 2
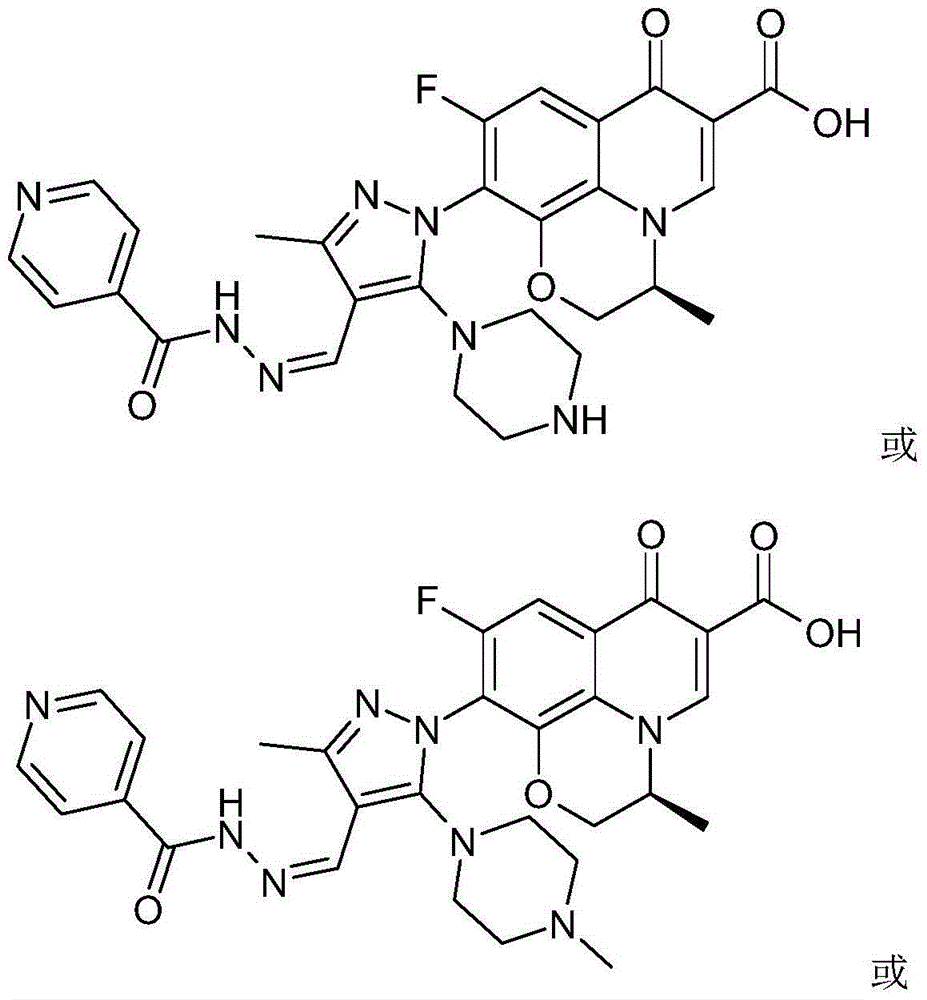
[0055] The chiral 7-(piperazine substituted pyrazole aldehyde isoniazid hydrazone) fluoroquinolone carboxylic acid derivative in this example is (S)-3-methyl-9-fluoro-10-[3-methyl- 4-(pyridine-4-carboxhydrazino)ylidenemethyl-5-(4-methyl-piperazin-1-yl)-pyrazol-1-yl]-2,3-dihydro-[1, 4] oxazino[2,3,4-ij]quinolin-7(4H)-one-6-carboxylic acid, whose chemical structural formula is:
[0056]
[0057] That is, R in formula (I) 1 is methyl, R 2 for a hydrogen atom.
[0058] The preparation method of the chiral 7-(piperazine substituted pyrazole aldehyde isoniazid hydrazone) fluoroquinolone carboxylic acid derivative of this example is: take 1.0 g (2.0 mmol) of (S)-3-methyl- 9-fluoro-10-[3-methyl-5-chloro-4-(pyridine-4-formylhydrazino)ylidenemethyl-pyrazol-1-yl]-2,3-dihydro-[1, 4] oxazino[2,3,4-ij]quinolin-7(4H)-one-6-carboxylic acid (VI) and 0.4g (4.0mmol) of anhydrous methylpiperazine, add 20ml of anhydrous Reflux reaction in acetonitrile for 16h, left overnight; The resulting...
Embodiment 3
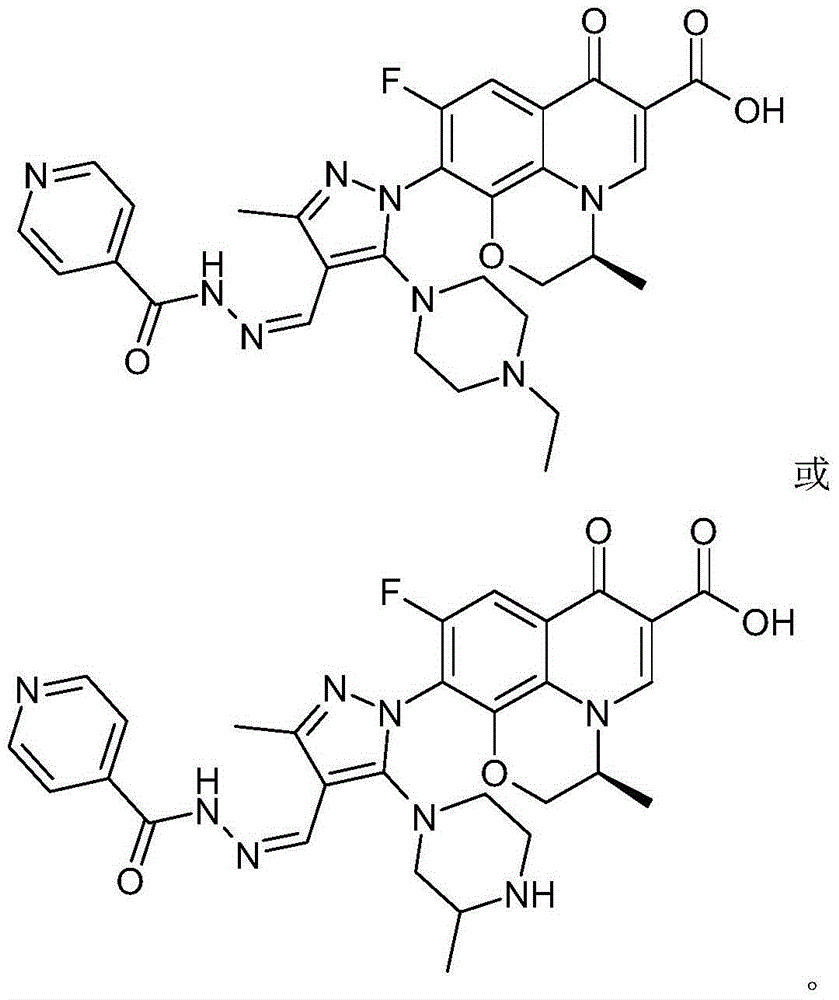
[0060] The chiral 7-(piperazine substituted pyrazole aldehyde isoniazid hydrazone) fluoroquinolone carboxylic acid derivative in this example is (S)-3-methyl-9-fluoro-10-[3-methyl- 4-(pyridine-4-carboxhydrazino)ylidenemethyl]-5-(4-ethyl-piperazin-1-yl)-pyrazol-1-yl]-2,3-dihydro-[1 ,4]oxazino[2,3,4-ij]quinolin-7(4H)-one-6-carboxylic acid, whose chemical structural formula is:
[0061]
[0062] That is, R in formula (I) 1 is ethyl, R 2 for a hydrogen atom.
[0063] The preparation method of the chiral 7-(piperazine substituted pyrazole aldehyde isoniazid hydrazone) fluoroquinolone carboxylic acid derivative of this example is: take 1.0 g (2.0 mmol) of (S)-3-methyl- 9-fluoro-10-[3-methyl-5-chloro-4-(pyridine-4-formylhydrazino)ylidenemethyl-pyrazol-1-yl]-2,3-dihydro-[1, 4] oxazino[2,3,4-ij]quinolin-7(4H)-one-6-carboxylic acid (VI) and 0.46g (4.0mmol) of anhydrous ethylpiperazine, add 20ml of anhydrous Reflux reaction in acetonitrile for 24h, left overnight; The resulting s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com