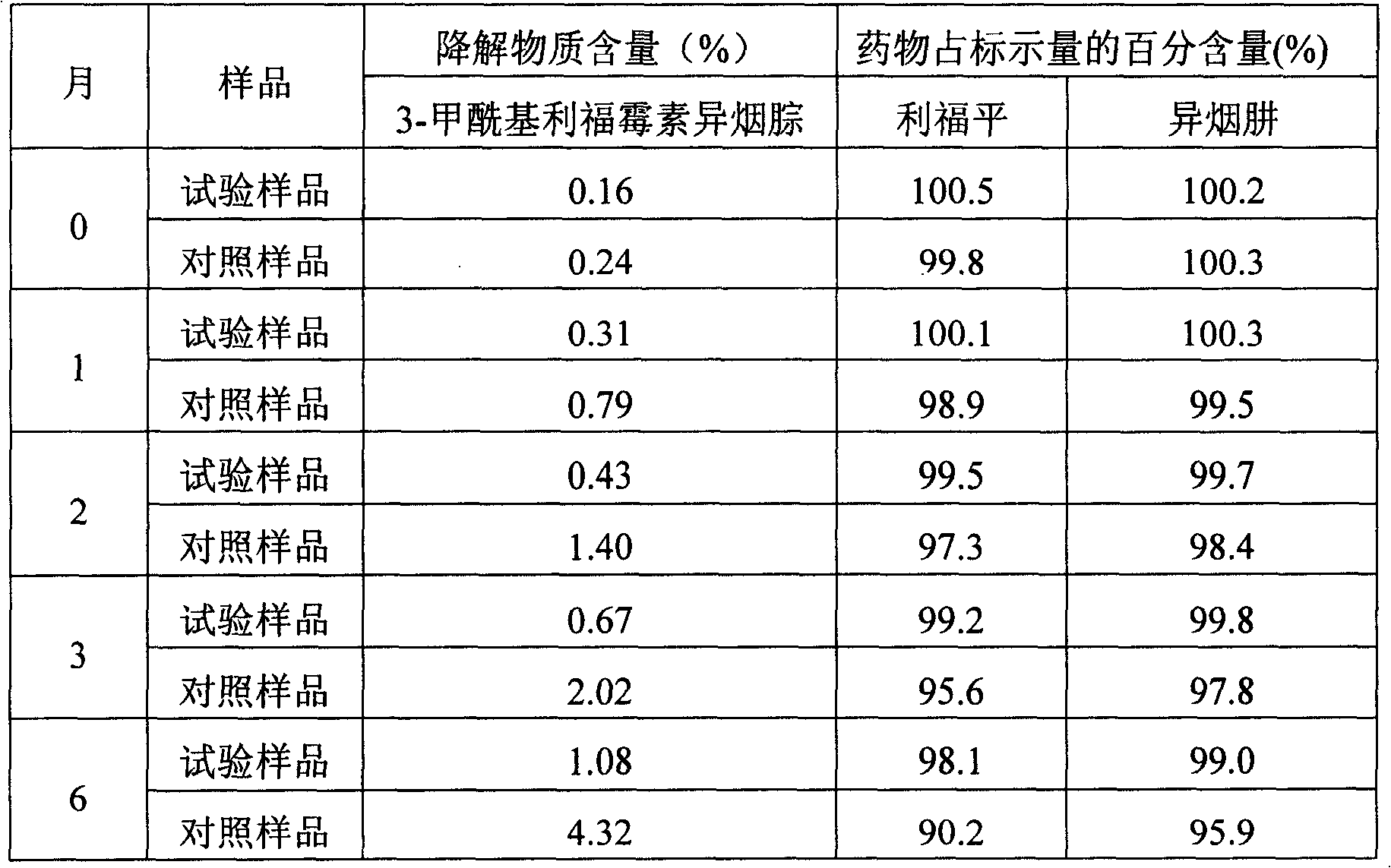
Pharmaceutical composition comprising rifamoin and isoniazid
A technology of isoniazid and rifampicin, applied in the field of pharmaceutical compositions containing rifampicin and isoniazid, can solve the problems of high cost, complicated operation and the like, and achieve the effects of convenient operation, simple process and increased stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1 Rifampicin and isoniazid compound film-coated tablets
[0032] 1000 tablets prescription:
[0033] Rifampicin 300g
[0034] Isoniazid 150g
[0035] Potassium dihydrogen phosphate 6g
[0036] Dipotassium hydrogen phosphate 12g
[0037] Microcrystalline cellulose 40g
[0038] Povidone 10g
[0039] Croscarmellose Sodium 7.5g
[0041] Preparation method: pass rifampicin, isoniazid, microcrystalline cellulose, povidone, croscarmellose sodium, potassium dihydrogen phosphate, and dipotassium hydrogen phosphate through an 80-mesh sieve, and weigh according to the prescription Take the above powder and 4g of magnesium stearate and mix them uniformly, take the dry granules with a dry granulation mechanism, take the dry granules and 2g of magnesium stearate and mix them evenly, and then press the tablets to control the tablet weight to 520mg-540mg. The plain tablets are film-coated with Opadry coating material.
Embodiment 2
[0042] Example 2 Rifampicin, isoniazid and pyrazinamide compound film-coated tablets
[0043] 1000 tablets prescription:
[0044] Rifampicin 120g
[0045] Isoniazid 80g
[0046] Pyrazinamide 250g
[0047] Citric acid 2g
[0048] Disodium hydrogen phosphate 9g
[0049] Microcrystalline cellulose 45g
[0050] Povidone 10g
[0051] Croscarmellose Sodium 8g
[0052] Magnesium stearate 5g
[0053] Preparation method: pass the raw materials and auxiliary materials through an 80 mesh screen, weigh 3g magnesium stearate and weigh the other raw materials and auxiliary materials according to the prescription, mix them, use a dry granulation mechanism to take dry particles, take dry particles and 2g hard Magnesium fatty acid is mixed uniformly and compressed into tablets to control the tablet weight between 520mg and 540mg. The plain tablets are film-coated with Opadry coating material.
Embodiment 3
[0054] Example 3 Compound capsules of rifampicin, isoniazid and pyrazinamide
[0055] 1000 capsules prescription:
[0056] Rifampicin 60g
[0057] Isoniazid 40g
[0058] Pyrazinamide 125g
[0059] Povidone 5g
[0060] Acetic acid 0.1g
[0061] Ammonium acetate 5g
[0062] Pregelatinized starch 15g
[0063] Magnesium stearate 5g
[0064] Preparation method: Weigh the prescription amount of povidone, acetic acid, and sodium acetate to dissolve in 50ml of water to make an adhesive. Pass the solid raw materials and auxiliary materials through an 80-mesh screen respectively, weigh the raw materials and auxiliary materials except magnesium stearate according to the prescription, mix them, and wet granulate with the prepared binder. After drying, take the dry granules and 5g hard Mix the magnesium fatty acid and fill the No. 2 capsule.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com