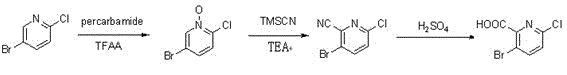Synthesis method of 3-bromo-6-chloropyridyl-2-formic acid
A synthesis method and chloropyridine technology, applied in directions such as organic chemistry, can solve problems such as environmental pollution, unsuitability for large-scale industrial production, high price, etc., and achieve the effect of reducing cost, low toxicity, safety and large-scale industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Step (1): Add trifluoroacetic anhydride (47.6 ml, 336 mmol) to 3-bromo-6-chloropyridine (32.4 g, 168 mmol) and urea peroxide (33.2 g, 350 mmol) under ice-bath cooling ) in chloroform (200 ml) solution. After the addition was complete, the reaction solution was stirred for 10 hours. The reaction temperature was 10°C. After the reaction was complete, saturated sodium thiosulfate solution was added and the solution was stirred for 40 minutes. The solution was separated into layers, and the aqueous phase was extracted twice with chloroform. The organic phases were combined and washed with saturated sodium bicarbonate solution and saturated sodium chloride solution respectively. The organic phase was dried with anhydrous sodium sulfate and spin-dried by a rotary evaporator to obtain a mixture. Ethyl acetate (200 ml) was added to the mixture and filtered with filter paper. The obtained solid was washed with ether and dried to obtain a yellow 3-bromo-6-chloropyridine nitro...
Embodiment 2
[0042] The main difference between this embodiment and embodiment 1 lies in step (1) and step (3).
[0043] Step (1): The reaction temperature in this example is 25°C.
[0044] Step (3): Dissolve 3-bromo-6-chloropyridine-2-cyanide (10 g, crude product) in 100 ml of 90% H 2 SO 4 solution, and the mixture was stirred at 150 °C for 4 hours. After the mixture had cooled to room temperature, it was poured onto 500 g of ice. After the mixture was filtered, the resulting solid was washed with water and dried to give 3-bromo-6-chloropyridine-2-carboxylic acid (8 g, 29.6% overall yield over two steps).
[0045] Its compound spectral figure characterizes the parameter of 3-bromo-6-chloropyridine-2-carboxylic acid as follows:
[0046] 1 HNMR (400 MHz, DMSO-d6): 8.02 (d, 1 H, J=4.8 Hz), 9.02 (d, 1 H, J=4.8 Hz).
Embodiment 3
[0048] The main difference between this embodiment and embodiment 1 lies in step (1) and step (3).
[0049] Step (1): The reaction temperature in this example is 25°C.
[0050] Step (3): Dissolve 3-bromo-6-chloropyridine-2-cyanide (10 g, crude product) in 100 ml of 90% H 2 SO 4 solution, and the mixture was stirred at 180 °C for 3 hours. After the mixture had cooled to room temperature, it was poured onto 500 g of ice. After the mixture was filtered, the resulting solid was washed with water and dried to give 3-bromo-6-chloropyridine-2-carboxylic acid (8.8 g, 32.6% overall yield over two steps).
[0051] Its compound spectral figure characterizes the parameter of 3-bromo-6-chloropyridine-2-carboxylic acid as follows:
[0052] 1 HNMR (400 MHz, DMSO-d6): 8.02 (d, 1 H, J=4.8 Hz), 9.02 (d, 1 H, J=4.8 Hz).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com