Method for synthesizing 2-acetyl-1, 10-phenanthroline (3)
A technology of phenanthroline and acetyl, which is applied in the field of synthesizing 2-acetyl-1, can solve the problems of low reaction selectivity, large consumption of hydrochloric acid, difficult separation, etc., to improve purification efficiency, increase reactivity, and low cost Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
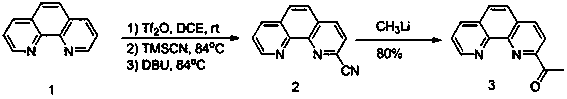
[0024] The method for preparing 2-acetyl-1,10-phenanthroline (3), comprising adding 1,10-phenanthroline and dichloroethane to a container at a mass ratio of 1:50, and then placing the container in an ice-water bath Under stirring, and add trifluoromethanesulfonic anhydride, the mass ratio of the addition amount of trifluoromethanesulfonic anhydride and 1,10-phenanthroline is 1:1, then the container is placed at room temperature and stirred for 1 hour, and trimethylsulfonic acid is added Cyanosilane, the mass ratio of trimethylsilyl cyanide to 1,10-phenanthroline is 4:1, then the container is heated to 84°C, and stirring is continued for 4 hours, and finally 1,8-diazepine is added Dicycloundec-7-ene (DBU), the mass ratio of 1,8-diazabicycloundec-7-ene (DBU) to 1,10-phenanthroline is 0.5:1 , stirred at 84°C for 17 hours, cooled the mixture to room temperature, quenched with saturated sodium bicarbonate solution, extracted with dichloromethane, washed with saturated sodium chlori...
Embodiment 2
[0026] Referring to Example 1, the method for preparing 2-acetyl-1,10-phenanthroline (3) includes adding 1,10-phenanthroline and dichloroethane to the container at a mass ratio of 1:100, and then The container was placed in an ice-water bath and stirred, and trifluoromethanesulfonic anhydride was added, and the mass ratio of the amount of trifluoromethanesulfonic anhydride to 1,10-phenanthroline was 1: 4, and then the container was stirred at room temperature for 1 hour, add trimethylsilyl cyanide, the mass ratio of the amount of trimethylsilyl cyanide to 1,10-phenanthroline is 2:1, then the container is warmed up to 84 ° C, continue to stir for 4 hours, and finally add 1, The amount of 8-diazabicycloundec-7-ene (DBU), 1,8-diazabicycloundec-7-ene (DBU) and the quality of 1,10-phenanthroline The ratio is 3:1, stirred at 84°C for 17 hours, cooled the mixture to room temperature, quenched with saturated sodium bicarbonate solution, extracted with dichloromethane, washed with satu...
Embodiment 3
[0028]Reference Example 1, the method for preparing 2-acetyl-1, 10-, phenanthroline (3), including adding 1, 10-phenanthroline and dichloroethane to the container at a mass ratio of 1:75, and then Place the container under an ice-water bath for stirring, and add trifluoromethanesulfonic anhydride, the mass ratio of the amount of trifluoromethanesulfonic anhydride to 1,10-phenanthroline is 1:2, and then place the container at room temperature to stir For 1 hour, add trimethylsilyl cyanide, the mass ratio of the amount of trimethylsilyl cyanide to 1,10-phenanthroline is 5:1, then the container is heated to 84°C, and stirring is continued for 4 hours, and finally 1 , 8-diazabicycloundec-7-ene (DBU), the amount of 1,8-diazabicycloundec-7-ene (DBU) and 1,10-phenanthroline The mass ratio is 2:1, stirred at 84°C for 17 hours, cooled the mixture to room temperature, quenched with saturated sodium bicarbonate solution, extracted with dichloromethane, washed with saturated sodium chlori...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com