Preparation method of furilazole
A technology of furoxazil and furanformaldehyde, which is applied in the direction of organic chemistry, can solve the problems of high requirements for reaction conditions, low yield, and complicated methods, and achieve the effects of simple operation, high yield, and reduced side reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
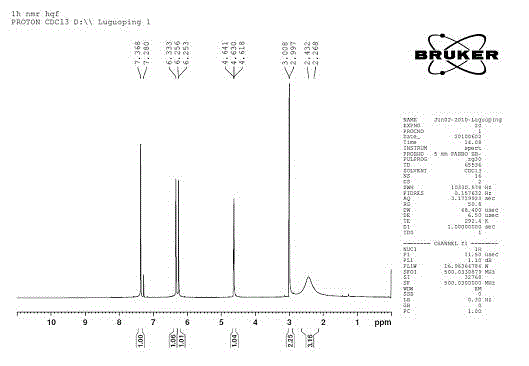
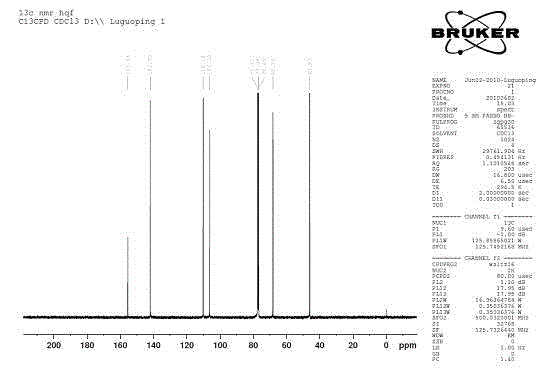
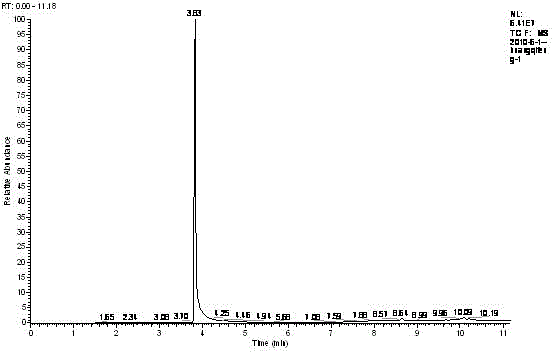
Image
Examples
preparation example Construction
[0019] The preparation method of furoclofenazole of the present invention, its steps are as follows:
[0020] The first step: with ZnI 2 、CuI 2 , Any one of KI Lewis acid catalysts is a catalyst, adding furanformaldehyde and trimethylsilylnitrile, wherein the molar ratio of furfuraldehyde and trimethylsilylnitrile is 1:1.0~1.5, and the reaction temperature is 5~20°C , The reaction time is 1.0~4.0h, and the reaction produces а-(2-furan)-а-trimethylsiloxyacetonitrile.
[0021] The second step: using one of ether and tetrahydrofuran as a solvent, reduce а-(2-furan)-а-trimethylsiloxyacetonitrile with lithium aluminum hydride, react under reflux for 1.0~3.0h, and use acetonitrile, methanol Recrystallization from other solvents gave а-aminomethyl-2-furanmethanol.
[0022] The third step: using any solvent in benzene, ethyl acetate, chloroform, and toluene as an entrainer, add а-aminomethyl-2-furyl methanol, toluenesulfonic acid, acetone, wherein а-aminomethyl- The molar ratio of...
Embodiment 1
[0025] The first step: add 0.10mol (CH 3 ) 3 SiCN and 30 mg ZnI 2 ;Slowly add furfural formaldehyde dropwise, the molar ratio of the two is 1:1.0, after the dropwise addition, continue to react at 5~10°C for 1h; after the reaction is finished, filter with diatomaceous earth to remove ZnI 2 Solid, the product obtained is yellow oily liquid A.
[0026] Step 2: Add 100ml of anhydrous ether to a 250ml four-neck flask, and then add 2.09gLiAlH after the temperature drops to 0°C 4 , slowly add 0.1mol liquid A dropwise, and react under reflux conditions for 1 h after the dropwise addition. Then react at room temperature for 2 h, filter with suction, wash the filter cake with ether, combine the filtrates, and wash with anhydrous MgSO 4 After drying, a yellow solid was obtained by rotary evaporation, and the filter cake and the yellow solid obtained by rotary evaporation were heated and dissolved with 100ml of tetrahydrofuran and filtered with suction, and the filter cake was washed...
Embodiment 2
[0054] Reaction step is exactly the same as embodiment 1, and difference: select CuI for the first step 2 It is a Lewis acid catalyst, the molar ratio of the reactants is 1:1.3, the reaction temperature is 10~15°C, and the reaction time is 2.5h; in the second step, tetrahydrofuran is selected as the reaction solvent, the reaction time is 2h, and acetonitrile is used for recrystallization; the third step Use ethyl acetate as the entrainer, the molar ratio of the reactants is 1:1.3, and the reflux reaction time is 2.5h; in the fourth step, triethylamine is selected as the acid-binding agent, and the molar ratio of the reactants is 1:1.2; the reaction temperature The temperature is -5~0℃, the reaction time is 10h, and benzene is used as the recrystallization solvent.
[0055] The confirmation method of the compound of this article embodiment is the same as that of Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com