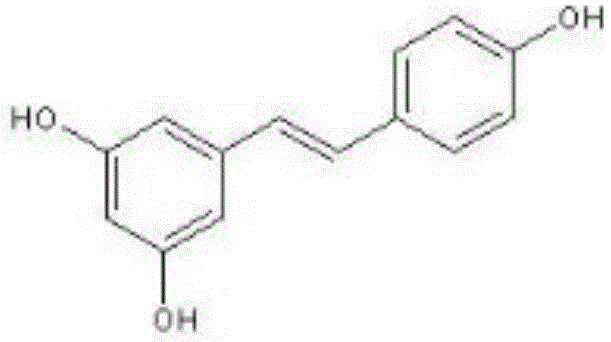
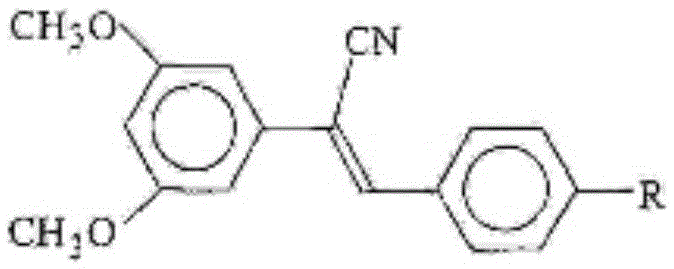
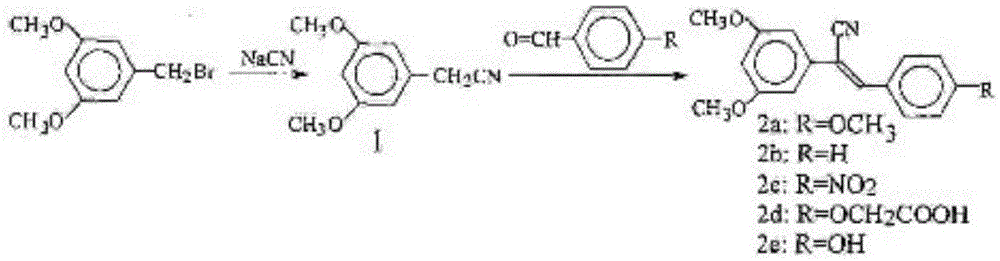
Cyano-containing resveratrol analogue and preparation method and purpose thereof
A technology for resveratrol and analogs, applied in the field of cyano-containing resveratrol analogs and their preparation, can solve the problems of inconvenient operation, limited application, environmental pollution and the like, and achieves reduction of health damage and application scope. Wide and environmentally friendly effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1: Preparation of Z-2-(3,5-dimethoxyphenyl)-3-(4-fluorophenyl)acrylonitrile
[0042] Synthesis of intermediate product 3,5-dimethoxybenzoic acid methyl ester 2
[0043] 14.231g (0.092mol) of 3,5-dihydroxybenzoic acid 1, 26.021g (0.189mol) of potassium carbonate and 100mL of acetone in a 500mL two-necked flask, use a constant pressure dropping funnel to slowly and uniformly add 17mL of dimethyl sulfate; about After 30 minutes of dropping, start heating and reflux for 4 hours; use thin layer chromatography to track and detect, and cool to room temperature after the reaction; filter, wash the filter residue with acetone and combine the filtrate, dry with anhydrous sodium sulfate, evaporate the solvent under reduced pressure, and dry to obtain compound 2; The yield was 90.7%, and the melting point was 41~43℃. 1 HNMR(300MHz, CDCl 3 ): δ7.18 (d, J = 2.3 Hz, 2H), 6.64 (t, J = 2.3 Hz, 1H), 3.90 (s, 3H), 3.82 (s, 6H).
[0044] Synthesis of the intermediate product 3,5-dimethox...
Embodiment 2
[0053] Example 2: Preparation of Z-2-(3,5-dimethoxyphenyl)-3-(4-chlorophenyl)acrylonitrile 6b, the reaction equation is as follows:
[0054]
[0055] Using the method similar to the synthesis of the intermediate product in Example 1, take 0.01mol of 3,5-dimethoxybenzeneacetonitrile (5), 0.01mol of 4-chlorobenzaldehyde and 20mL of methanol into a 50mL three-necked flask, stir and warm up to 60℃, add methanol Sodium 0.005mol, constant temperature reaction 4-6h, TLC tracking detection, after the reaction is completed, cool to room temperature, filter, wash with water, dry, recrystallize with methanol to obtain a white solid, the yield is 73.9%, the melting point is 114-116℃ . 1 HNMR(300MHz, CDCl 3 ): δ7.82(d,J=8.6Hz,2H), 7.46(d,J=4.6Hz,2H), 7.42(s,1H), 6.79(d,J=2.1Hz,2H), 6.49(dd , J=6.0, 3.9 Hz, 1H), 3.85 (s, 6H).
Embodiment 3
[0056] Example 3: Preparation of Z-2-(3,5-dimethoxyphenyl)-3-(4-bromophenyl)acrylonitrile 6c, the reaction equation is as follows:
[0057]
[0058] Using the method similar to the synthesis of intermediate products in Example 1, take 0.01mol 3,5-dimethoxybenzeneacetonitrile (5), 0.01mol 4-bromobenzaldehyde and 20mL methanol into a 50mL three-necked flask, stir and raise the temperature to 60℃, add methanol Sodium 0.005mol, constant temperature reaction 4-6h, TLC tracking detection, after the reaction is completed, cool to room temperature, filter, wash with water, dry, recrystallize with methanol to obtain a pale yellow solid, the yield is 66.3%, the melting point is 150-152 ℃. 1 HNMR(300MHz, CDCl 3 )δ7.91(d,J=5.4Hz,2H),7.48(s,1H),7.16(t,J=8.6Hz,2H), 6.79(d,J=2.2Hz,2H), 6.50(t, J = 2.2 Hz, 1H), 3.85 (s, 6H). 13CNMR (75MHz, CDCl3): δ 161.27, 141.08, 136.14, 132.45, 132.23, 130.71, 124.95, 117.72, 112.33, 104.32, 101.43, 55.57. IR(KBr)cm -1 : 2835(-OCH3), 2220(-CN), 858(-C=C-H)....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com