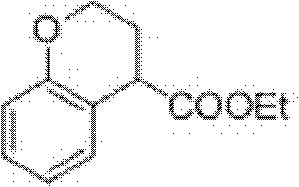
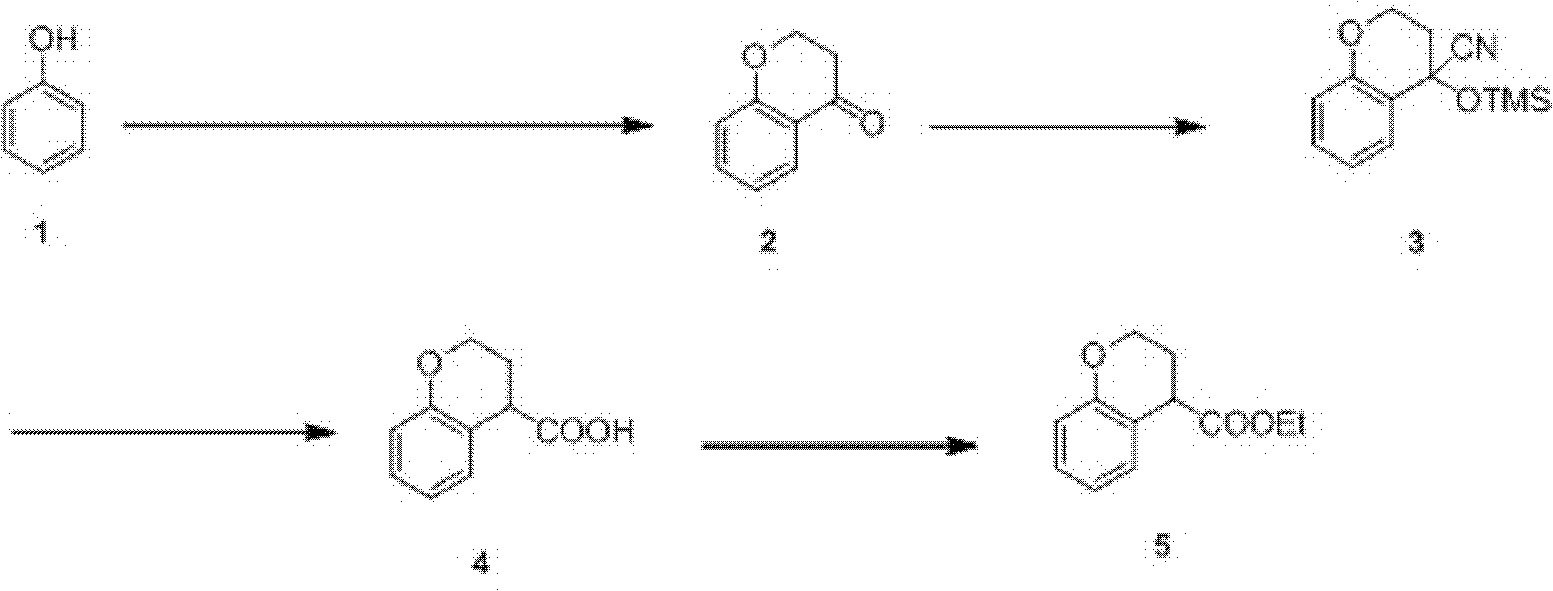
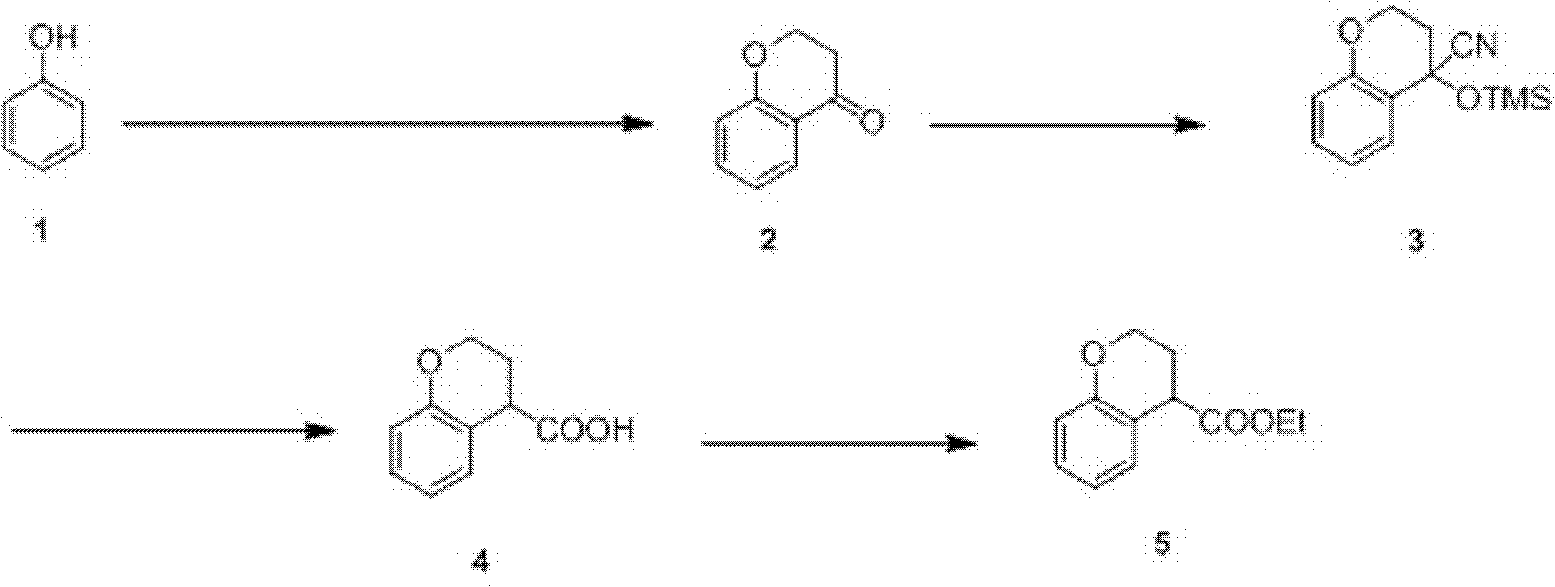
Preparation process of ethyl chromane-4-formate
A technology of ethyl formate and preparation process, applied in the field of preparation of organic compounds, can solve problems such as high cost and low yield, and achieve the effects of cost reduction and yield improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] The preparation technology of chroman-4-ethyl carboxylate is made up of following four steps:
[0026] Step 1: Friedel-Crafts acylation, cyclization
[0027] (1) Under a nitrogen environment, put 1.6kg of compound phenol of formula 1 into a 20L four-necked bottle equipped with a condenser and mechanical stirring, that is, 17.0mol, and 10L of dry nitrobenzene as a solvent, and heat to dissolve completely;
[0028] (2) reactant is cooled to room temperature subsequently, drop into 2.2kg of 3-chloropropionyl chlorides, i.e. 17.0mol;
[0029] (3) A total of 5 kg of anhydrous aluminum trichloride was added into the reactant within 1 hour in ten times, and the hydrogen chloride gas produced was absorbed by sodium hydroxide solution, and the reactant was stirred at 40-45°C for 5 minutes after stirring for 1 hour. Hour, track with HPLC, until reaction finishes;
[0030] (4) After the reactant was cooled to room temperature, add 25 kg of ice and 6 L of concentrated hydrochlori...
Embodiment 2
[0051] Embodiment 2 is basically the same as Embodiment 1, and the only difference is that step 1 is different, as follows:
[0052] Friedel-Crafts acylation, cyclization
[0053] (1) Under a nitrogen atmosphere, put 0.16kg of compound phenol of formula 1 into a 2L four-neck flask equipped with a condenser and mechanical stirring, that is, 1.7mol, add 1L of dry 1,2-dichloroethane as a solvent, and heat to dissolve completely ;
[0054] (2) The reactant is cooled to room temperature, and 0.22kg of 3-chloropropionyl chloride is dropped into, i.e. 1.7mol;
[0055] (3) 0.5 kg of anhydrous aluminum trichloride was added in 5 times within 1 hour, the hydrogen chloride gas generated was absorbed with sodium hydroxide solution, the reactants were stirred for 1 hour and then reacted at 40-45°C for 5 hours, followed by HPLC , until the end of the reaction.
[0056] (4) The reactant is cooled to room temperature, add 2.5kg ice and 0.6L concentrated hydrochloric acid, stir for 30 minut...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com