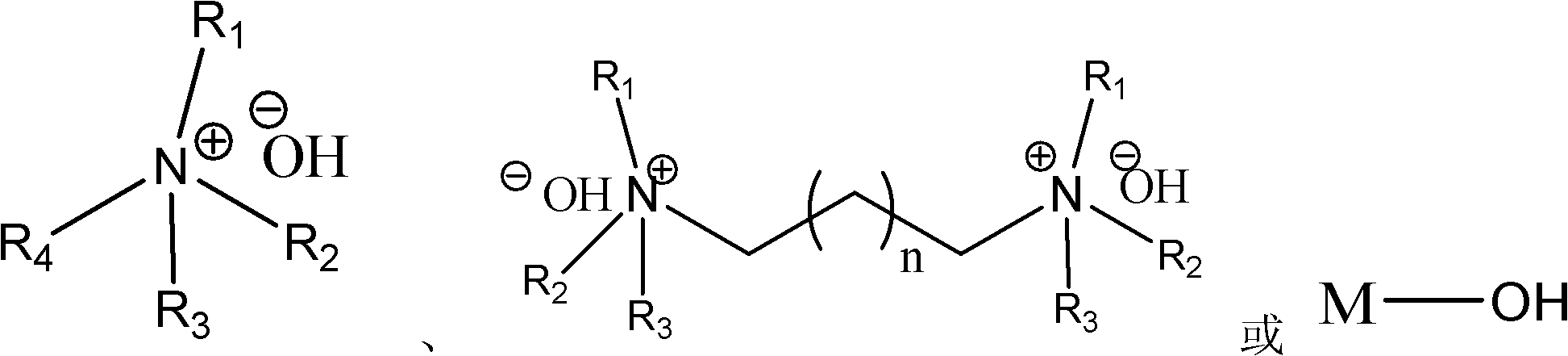
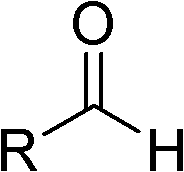
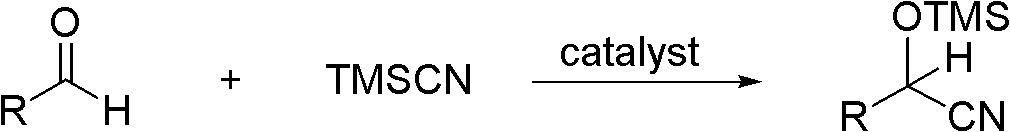Highly active catalyst used for hydrosilation reaction of aldehyde and trimethylsilyl cyanide
A technology of trimethylsilyl cyanide and high-activity catalysts, which is applied in the field of high-activity catalysts, can solve the problems of using organic solvent reaction conditions, large amount of catalysts, nonconformities, etc., and achieves wide application range, simple operation, and mild reaction conditions. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Preparation of isobutyraldehyde cyanohydrin siloxane
[0021] Add 2.3mL (0.025mol) of freshly distilled isobutyraldehyde to a 25mL one-necked flask, and then add 0.46μL (5.0×10 -8 mol) with a mass fraction of 1% (CH 3 ) 4 Add 3.4mL (0.025mol) trimethylsilyl cyanide (the molar ratio of catalyst, isobutyraldehyde and trimethyl silyl cyanide is 1: 500000: 500000) to the NOH aqueous solution with stirring at room temperature, the reaction is violent (Note : The heat released in the reaction is released naturally by itself), and the reaction ends in 10 minutes. 1 The conversion was determined to be 100% by H NMR.
Embodiment 2
[0023] Preparation of Benzaldehyde Cyanohydrin Silicon Ether
[0024] Add 53g (0.5mol) of freshly distilled benzaldehyde to a 250mL single-necked flask, and then add 0.36μL (1.0×10 -6 mol) with a mass fraction of 25% (CH 3 ) 4 Add 49.5 g (0.5 mol) of trimethylsilyl cyanide to NOH aqueous solution under room temperature and stirring state, the reaction is violent, TLC follows the reaction, after 20 minutes a small amount of sample is taken and sent to NMR, the calculated conversion rate is 98%.
Embodiment 3
[0026] Preparation of Cyclohexylformaldehyde Cyanohydrin Silicon Ether
[0027] Add 60mL (0.5mol) of freshly distilled cyclohexyl formaldehyde to a 250mL one-necked flask, then add 0.26g (1.0×10 -6 mol) n Bu 4 NOH solid, add 66.6mL (0.5mol) trimethylsilyl cyanide under stirring state at room temperature, the reaction is violent, after 10 minutes take a small amount and send it to NMR, the calculated conversion rate is 99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com