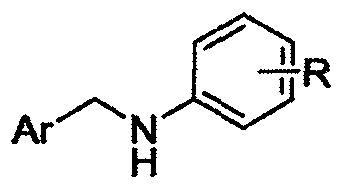
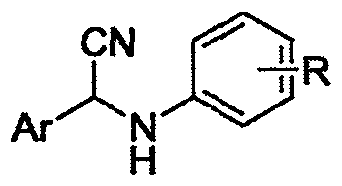
Alpha-cyaniding method of N-arylmethylaniline
A technology of arylmethylaniline and trimethylsilyl cyanide, which is applied in the field of organic chemical synthesis, can solve problems such as unfavorable industrial production, metal ion pollution of products, inapplicability to mass production, etc., achieve high yield, save industrial costs, The effect of a short reaction cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] A kind of α-cyanidation method of N-arylmethylaniline, its concrete steps are as follows:
[0024] (1) Take N-benzylaniline as raw material, N-benzylaniline mmol: TMSCN mmol: DDQ mmol: the ratio of ethyl acetate milliliter ratio 1: 2.0: 1.2: 4, first add in the reactor N-benzylaniline (91.6mg, 0.5mmol), was added solvent ethyl acetate (2mL), under stirring, then added trimethylsilyl cyanide (125μL, 1.0mmol), DDQ (136.2mg, 0.6mmol), and After finishing, at room temperature, the oxidative cyanation reaction was carried out with continuous stirring for 5 hours.
[0025] (2) After step (1) is completed, add saturated sodium thiosulfate solution to the reaction solution prepared in step (1) to quench, extract with ethyl acetate, combine organic phases, and dry over anhydrous magnesium sulfate. Suction filtration, rotary evaporation to concentrate the organic phase, the organic phase was purified by silica gel column chromatography, and eluted with the eluent, and the efflue...
Embodiment 2
[0028] A kind of α-cyanidation method of N-arylmethylaniline, its concrete steps are as follows:
[0029] (1) Take N-benzylaniline as raw material, N-benzylaniline mmol: TMSCN mmol: DDQ mmol: the ratio of DMF milliliter ratio 1: 2.0: 1.2: 4, add N- Benzylaniline (91.6mg, 0.5mmol), add the solvent DMF (2mL), under stirring, then add trimethylsilyl cyanide (125μL, 1.0mmol), DDQ (136.2mg, 0.6mmol) successively, after addition, at room temperature Under continuous stirring, the oxidative cyanation reaction was carried out for 5 hours.
[0030] (2) After step (1) is completed, add saturated sodium thiosulfate solution to the reaction solution prepared in step (1) to quench, extract with ethyl acetate, combine organic phases, and dry over anhydrous magnesium sulfate. Suction filtration, rotary evaporation to concentrate the organic phase, the organic phase was purified by silica gel column chromatography, and eluted with the eluent, and the effluent of the silica gel column chromat...
Embodiment 3
[0033] A kind of α-cyanidation method of N-arylmethylaniline, its concrete steps are as follows:
[0034] (1) Take N-benzylaniline as raw material, N-benzylaniline mmol: TMSCN mmol: DDQ mmol: the ratio of acetonitrile milliliter ratio 1: 2.0: 1.2: 4, add N-benzylaniline earlier in the reactor Benzylaniline (91.6mg, 0.5mmol), add the solvent acetonitrile (2mL), under stirring, then add trimethylsilyl cyanide (125μL, 1.0mmol), DDQ (136.2mg, 0.6mmol) successively, after addition, at room temperature Under continuous stirring, the oxidative cyanation reaction was carried out for 5 hours.
[0035] (2) After step (1) is completed, add saturated sodium thiosulfate solution to the reaction solution prepared in step (1) to quench, extract with ethyl acetate, combine organic phases, and dry over anhydrous magnesium sulfate. Suction filtration, rotary evaporation to concentrate the organic phase, the organic phase was purified by silica gel column chromatography, and eluted with the elu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com