2-cyanoindole substituted gem-difluoroolefin compound as well as preparation method and application thereof
A technology of gem-difluoroalkene and cyanoindole, which is applied in the field of 2-cyanoindole-substituted gem-difluoroalkene compound and its preparation, can solve the problems of substrate reaction limitations and achieve simple operation and mild reaction conditions , the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] The reaction equation is:
[0041]
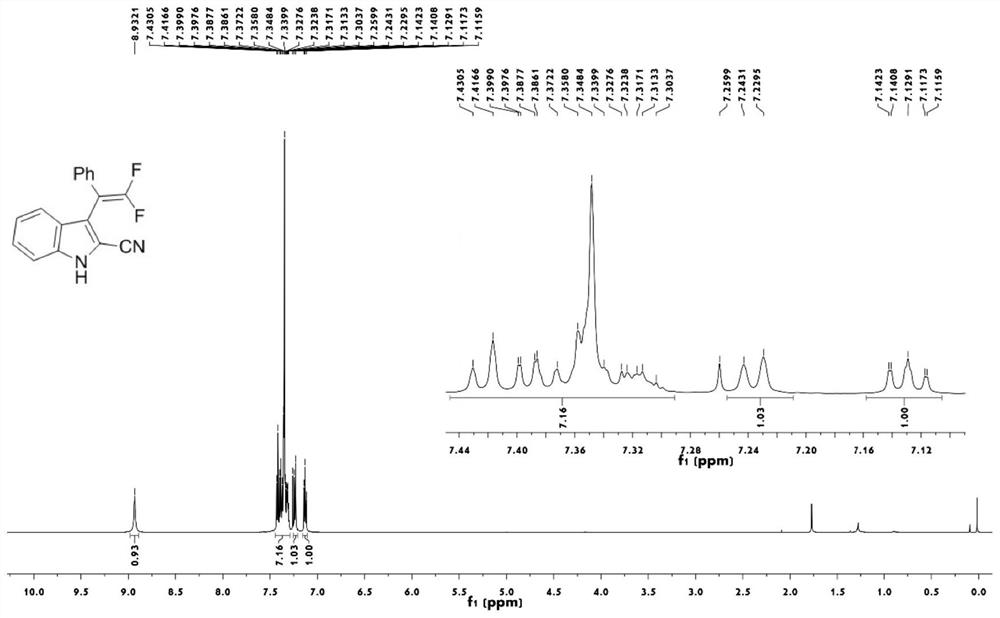
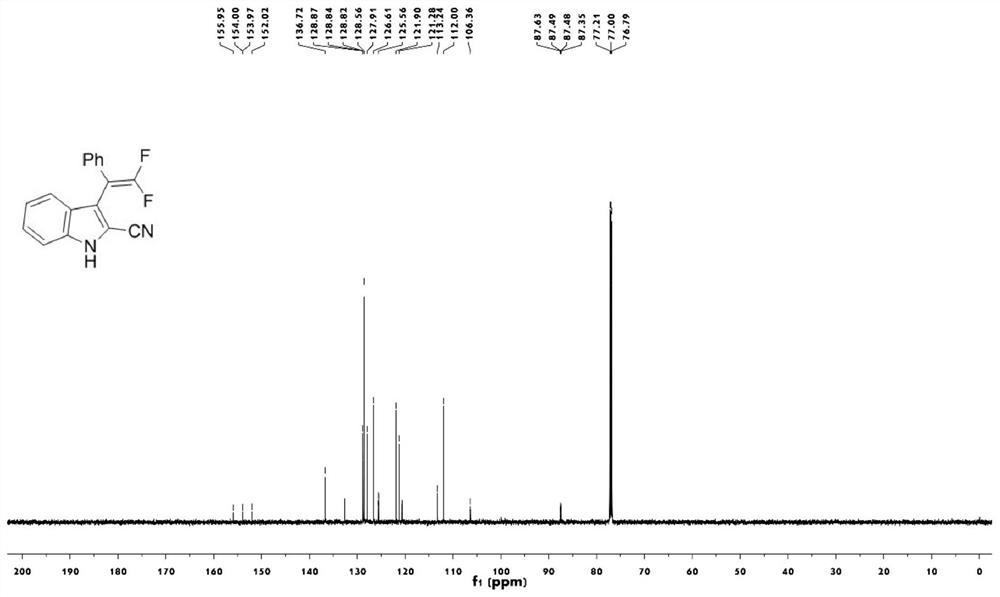
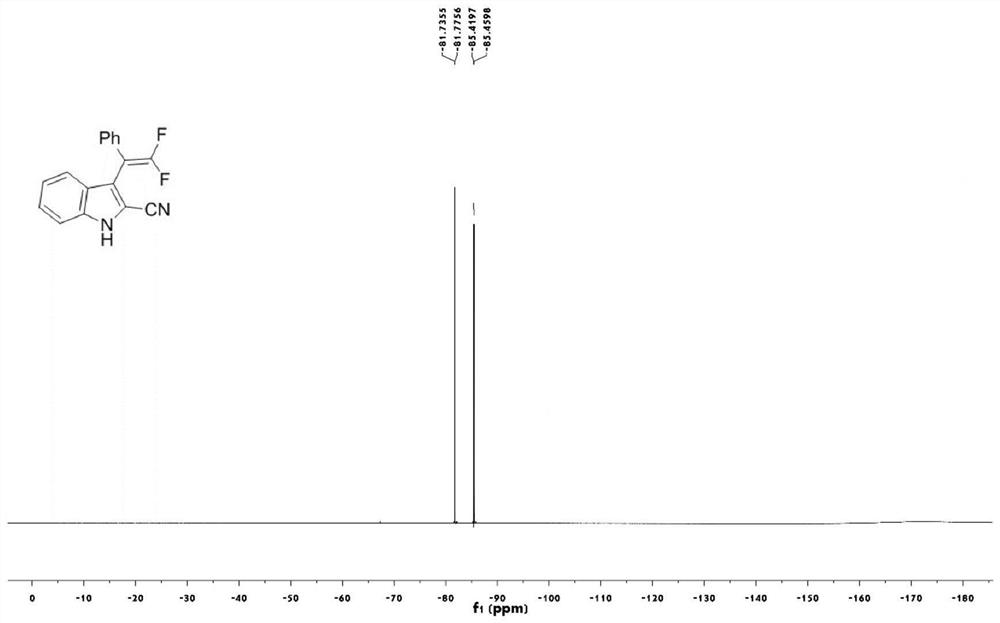
[0042] Take a 25mL round bottom flask, add trifluoromethylindolemethanol 2a (87.4mg, 0.3mmol), magnesium oxide (3mmol), scandium trifluoromethanesulfonate (0.06mmol), trimethylsilyl cyanide (0.9mmol) in sequence , anhydrous chlorobenzene (3mL), stirred at 120°C for 12 hours (thin plate chromatography tracked the reaction until the reaction was complete), cooled to room temperature after the reaction, filtered to remove insolubles, and the filtrate was spun and concentrated to remove the solvent, with a volume ratio of 25: A 1-10:1 mixed solution of petroleum ether and ethyl acetate was used as the eluent for gradient elution to obtain the target product 1a (63.1 mg, white solid, yield 75%).
[0043] figure 1 The proton nuclear magnetic resonance spectrum of compound 1a prepared for the present embodiment;
[0044] figure 2 The carbon nuclear magnetic resonance spectrum of the compound 1a prepared for the present embodiment;
[...
Embodiment 2
[0049] The reaction equation is:
[0050]
[0051] Take a 25mL round bottom flask, add trifluoromethylindolemethanol 2b (91.6mg, 0.3mmol), magnesium oxide (3mmol), scandium trifluoromethanesulfonate (0.06mmol), trimethylsilyl cyanide (0.9mmol) in sequence , anhydrous chlorobenzene (3mL), stirred at 120°C for 8 hours (thin plate chromatography tracked the reaction until the reaction was complete), cooled to room temperature after the reaction, filtered to remove insolubles, and the filtrate was spun and concentrated to remove the solvent, with a volume ratio of 25: A 1-10:1 mixed solution of petroleum ether and ethyl acetate was used as the eluent for gradient elution to obtain the target product 1b (70.6 mg, white solid, yield 80%).
[0052] 1 H NMR (400MHz, CDCl 3 )δ8.98(s,1H),7.36–7.30(m,6H),7.22(d,J=8.5Hz,1H),7.05(s,1H),2.37(s,3H); 13 C NMR (101MHz, CDCl 3 )δ153.9(dd, J=296.9,293.9Hz),135.2,132.7(dd,J=5.1,3.0Hz),128.8(dd,J=4.0,3.0Hz),128.6128.5,127.8,125.8(d,J = 2.0...
Embodiment 3
[0054] The reaction equation is:
[0055]
[0056] Take a 25mL round bottom flask, add trifluoromethylindolemethanol 2c (104.8mg, 0.3mmol), magnesium oxide (3mmol), scandium trifluoromethanesulfonate (0.06mmol), trimethylsilyl cyanide (0.9mmol) in sequence , anhydrous chlorobenzene (3mL), stirred at 120°C for 24 hours (thin plate chromatography followed the reaction until the reaction was complete), cooled to room temperature after the reaction, filtered to remove insolubles, and the filtrate was spun and concentrated to remove the solvent, with a volume ratio of 20: A 1-5:1 mixed solution of petroleum ether and ethyl acetate was used as the eluent for gradient elution to obtain the target product 1c (52.8 mg, white solid, yield 52%).
[0057] 1 H NMR (600MHz, CDCl 3 )δ9.41(s,1H),8.08–8.06(m,2H),7.45(d,J=9.3Hz,1H),7.36–7.29(m,5H),3.90(s,3H); 13 C NMR (151MHz, CDCl 3 )δ167.5, 154.0 (dd, J = 299.0, 293.7Hz), 139.1, 132.3 (dd, J = 4.6, 3.0Hz), 128.7 (t, J = 3.5Hz), 128.6, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com