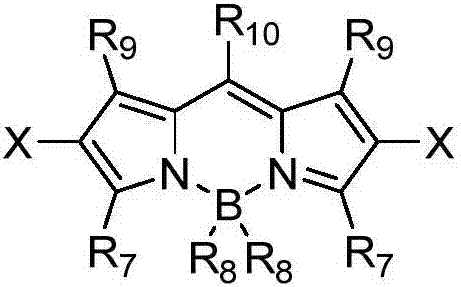
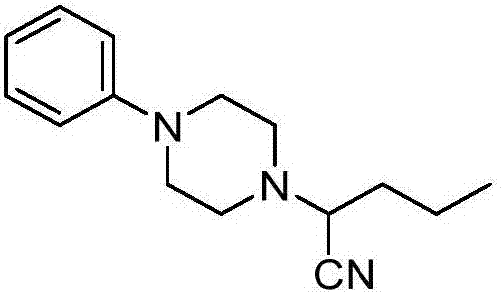
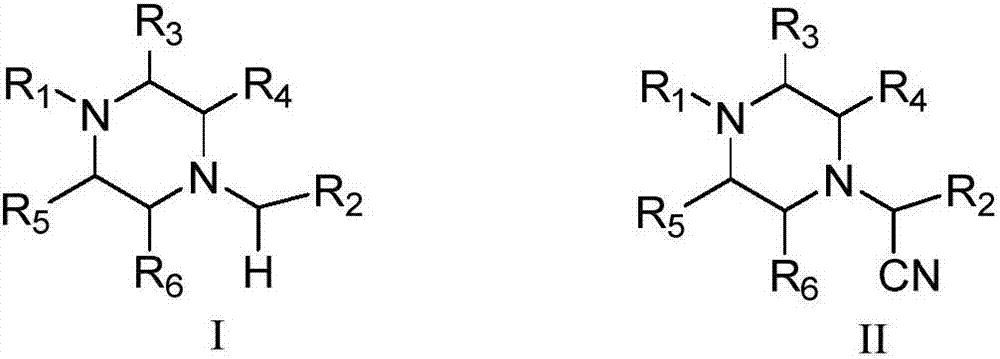Method for visible light catalytic selective cyanation of piperazine compound
A technology for catalyzing piperazine and compounds, which is applied in the field of selective cyanation, can solve the problems of high cost of noble metals, high toxicity, unsuitability for selective cyanation of piperazine drug molecules, and high synthesis cost, and achieves the scope of substrate application. Wide, less catalyst dosage, easy to operate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Preparation of 2-(4-phenylpiperazin-1-yl) valeronitrile of the following structural formula
[0019]
[0020] Multi-substituted BODIP-5 organic photocatalyst (1.80mg, 0.0025mmol), TMSCN (99mg, 1mmol), 1-butyl-4-phenylpiperazine (54mg, 0.25mmol), dichloromethane (3mL) were added to In the optical parallel reaction tube, turn on the 3W blue LED light, and stir the reaction at 30°C for 12 hours. After the reaction is completed, add 15 mL of saturated saline to the mixture, and then extract it three times with 15 mL of dichloromethane, collect the organic phase, and use Water Na 2 SO 4 Dry, filter with suction, remove the solvent with a rotary evaporator, and separate by column chromatography to obtain 47 mg of white solid 2-(4-phenylpiperazin-1-yl)valeronitrile, with a yield of 83%, and the characterization data are: 1 H NMR (400MHz, CDCl 3): δ7.22(t, J=8.0Hz, 2H), 6.88(t, J=8.4Hz, 2H), 6.82(t, J=7.2Hz, 1H), 3.50(t, J=8.0Hz, 1H ),3.23-3.12(m,4H),2.83-2.78(m,2H),2.61...
Embodiment 2
[0030] Preparation of 2-(4-(p-tolyl)piperazin-1-yl)valeronitrile of the following structural formula
[0031]
[0032] In the present embodiment, replace the 1-butyl-4-phenylpiperazine in embodiment 1 with the 1-butyl-4-(p-tolyl) piperazine of equimolar amount, the reaction time is extended to 15 hours, other The procedure was the same as in Example 1 to obtain 40 mg of white solid 2-(4-(p-tolyl)piperazin-1-yl)valeronitrile with a yield of 61%, and the characteristic data were: 1 H NMR (400MHz, CDCl 3 ): δ7.08(d, J=8.0Hz, 2H), 6.84(d, J=8.4Hz, 2H), 3.55(t, J=7.2Hz, 1H), 3.53-3.12(m, 4H), 2.88 -2.82(m,2H),2.66-2.61(m,2H),2.27(s,3H),1.82-1.74(m,2H),1.57-1.50(m,2H),0.98(t,J=7.2Hz ,3H)ppm; 13 C NMR (100MHz, CDCl 3 ): δ149.1, 129.79, 129.73, 117.1, 116.7, 57.7, 49.8, 49.7, 33.0, 20.5, 19.4, 13.5ppm; HRMS (m / z, ESI) theoretical value C 16 h 23 N 3 Na + [M+Na] + : 280.1790, the measured value is 280.1789.
Embodiment 3
[0034] Preparation of 2-(4-(2-methoxyphenyl)piperazin-1-yl)valeronitrile of the following structural formula
[0035]
[0036] In this example, replace 1-butyl-4-phenylpiperazine in Example 1 with equimolar amounts of 1-butyl-4-(2-methoxyphenyl)piperazine, other steps and implementation Same as Example 1, 50 mg of yellow oily matter 2-(4-(2-methoxyphenyl) piperazin-1-yl) valeronitrile was obtained, the yield was 73%, and the characterization data were: 1 H NMR (400MHz, CDCl 3 ):δ7.02-6.97(m,1H),6.91(d,J=4.4Hz,2H),6.87(d,J=8.0Hz,1H),3.86(s,3H),3.54(t,J= 7.6Hz,1H),3.12(s,4H),2.91-2.86(m,2H),2.70-2.65(m,2H),1.87-1.71(m,2H),1.62-1.45(m,2H),0.98 (t,J=7.2Hz,3H)ppm; 13 C NMR (100MHz, CDCl 3 ): δ152.4, 141.1, 123.18, 121.11, 118.3, 117.3, 111.6, 57.7, 55.5, 50.4, 50.0, 33.0, 19.4, 13.5ppm; HRMS (m / z, ESI) theoretical value C 16 h 23 N 3 NaO + [M+Na] + : 296.1739, the measured value is 296.1734.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com