Preparation method of topiroxostat
A technology of topicastat and an intermediate, which is applied in the field of pharmaceutical synthesis, can solve the problems of high price of 4-cyanopyridine nitrogen oxide, large environmental pollution, small supply and the like, and achieves low cost of raw materials, high purity, The effect of reducing usage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
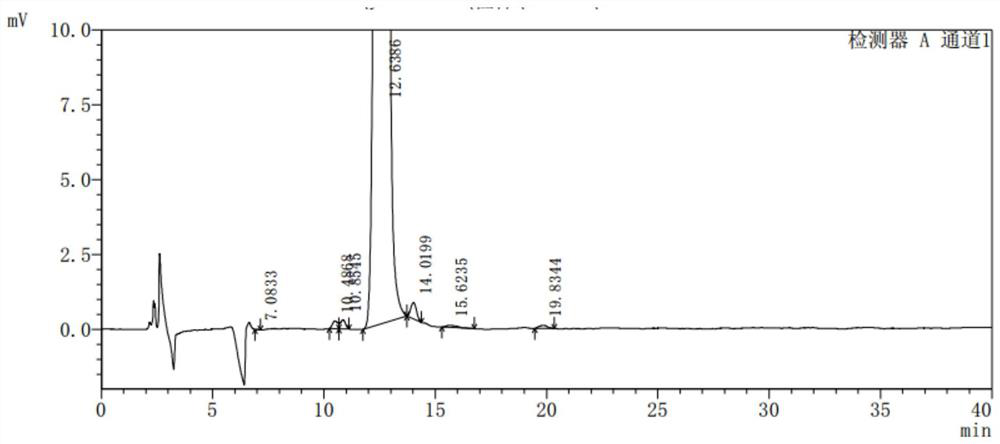
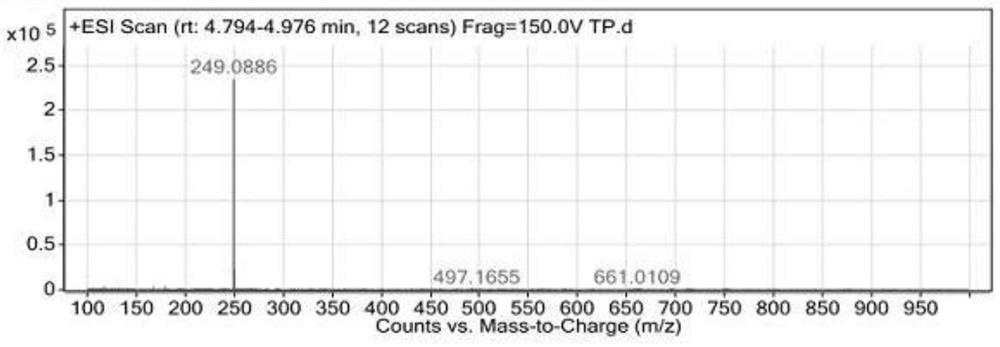
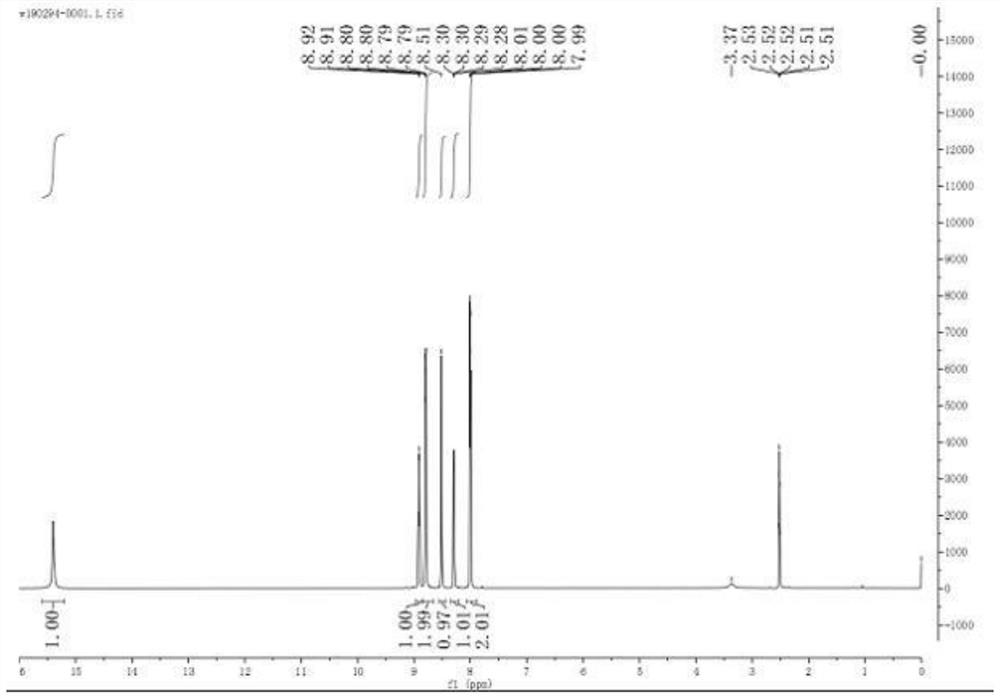
Image
Examples
Embodiment 1
[0062] A preparation method of Topicastat, comprising the steps of:
[0063] (1) Preparation of Intermediate 1:
[0064]
[0065] The starting material isonicotinic acid (20.0g, 0.16mol, 1.0eq) was dissolved in acetic acid (100mL), and hydrogen peroxide (54.4g, content 30%, 0.48mol, 3.0eq) was added at room temperature (about 25°C), The temperature was raised to 90°C for 8h. HPLC showed that there was no raw material left, stop heating, lower to room temperature, add 200 mL of acetone, crystallize for 1 h, filter, and rinse the filter cake twice with a small amount of acetone (100 mL in total). The obtained solid was air-dried at 45° C. for 10 h. 20.91 g of the product was obtained, yield: 93.9%.
[0066] (2) Preparation of Intermediate 2:
[0067]
[0068] Intermediate 1 (10.0g, 71.9mmol, 1.0eq) was dissolved in methanol (80mL), concentrated sulfuric acid (5.0g) was added dropwise, the reflux reaction temperature was controlled at 64°C, and the reflux reaction was c...
Embodiment 2
[0084] With the preparation method of topicastat described in embodiment 1, the difference is:
[0085] Step (1) Preparation of Intermediate 1:
[0086] 28.0 g of the starting material isonicotinic acid was dissolved in 100 mL of acetic acid, 60.2 g of hydrogen peroxide was added at room temperature, and the temperature was raised to 80° C. for 9 h. HPLC showed that there was no raw material left, stop heating, lower to room temperature, add 200 mL of acetone, crystallize for 1 h, filter, and rinse the filter cake twice with a small amount of acetone (100 mL in total). The obtained solid was air-dried at 45° C. for 10 h.
Embodiment 3
[0088] With the preparation method of topicastat described in embodiment 1, the difference is:
[0089] Step (2) Preparation of Intermediate 2:
[0090] Dissolve 18.0g of Intermediate 1 in 80mL of methanol, add 6.0g of concentrated sulfuric acid dropwise, control the reflux reaction temperature to 68°C, and reflux for 8 hours. TLC shows that there is a small amount of raw material remaining. Stop heating, cool down in an ice-water bath, and add sodium carbonate solid in batches , control the temperature not to exceed 20°C, adjust the pH to 7, filter, rinse the filter cake twice with a small amount of methanol, evaporate the methanol to dryness under reduced pressure at 45°C, dissolve the obtained solid in dichloromethane (100mL), and wash with 5 % sodium carbonate aqueous solution 15mL washed once, the dichloromethane phase was separated, dried with a little sodium sulfate, and evaporated to dryness under reduced pressure at 45°C to obtain the product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com