Alpha-cyanidation method of mono-alkyl substituted aniline
A monoalkyl and benzyl aniline technology, applied in the field of organic chemical synthesis, can solve the problems of unfavorable industrial production, lack of investigation of other substrates, unsuitable for mass production, etc., achieve high yield, save industrial costs, and shorten the reaction cycle short effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
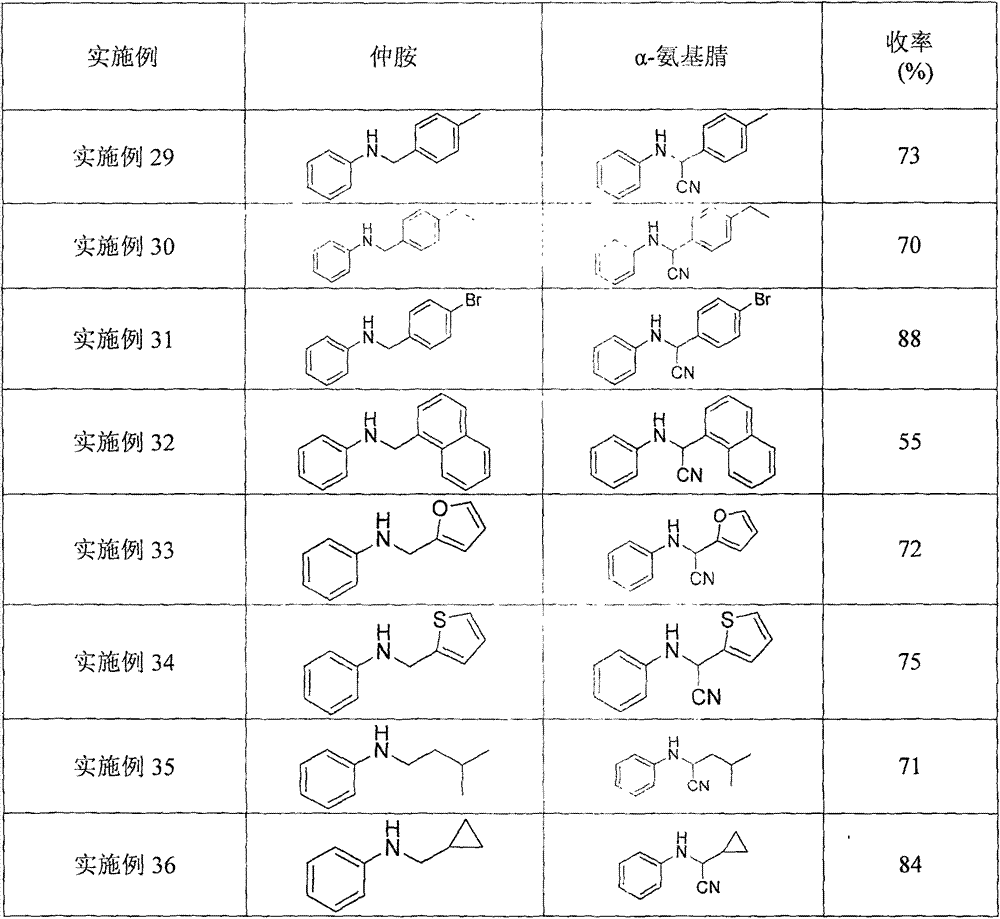
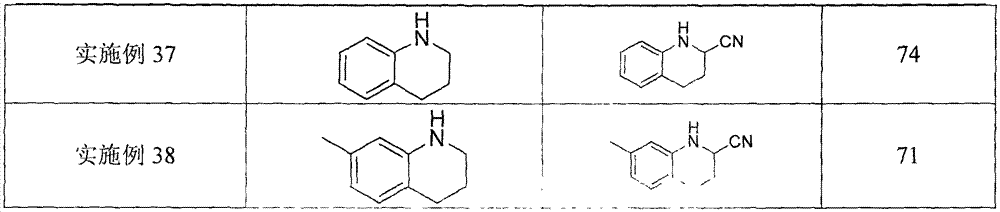
Examples
Embodiment 1
[0022] A kind of α-cyanation method of monoalkyl substituted aniline, its concrete steps are as follows:
[0023] (1) With N-benzylaniline as raw material, trimethylsilyl cyanide as cyanide reagent, ferrocene as catalyst, tert-butyl hydroperoxide as oxidant, according to N-benzylaniline mmol: trimethylsilyl Cyanogen mmol: ferrocene mmol: the ratio of tert-butyl hydroperoxide is 1: 2: 0.1: 2, and N-benzylaniline (91.6 mg, 0.5 mmol), ferrocene Iron (9.2 mg, 0.05 mmol), in the solvent dimethyl sulfoxide, under stirring, then add trimethylsilyl cyanide (125.1 μL, 1.0 mmol), tert-butyl hydroperoxide (148.0 μL, 1.0 mmol), After the addition, at 25°C, the catalytic oxidative cyanation reaction was carried out with continuous stirring for 4 hours.
[0024] (2) After the first (1) step is completed, the reaction solution of the α-aminonitrile compound prepared in the (1) step is quenched with saturated sodium bicarbonate and sodium thiosulfate solution, and dichloromethane is extracte...
Embodiment 2
[0027] A kind of α-cyanation method of monoalkyl substituted aniline, its concrete steps are with embodiment 1, wherein:
[0028] (1) With N-benzylaniline as raw material, trimethylsilyl cyanide as cyanide reagent, ferrocene as catalyst, tert-butyl hydroperoxide as oxidant, according to N-benzylaniline mmol: trimethylsilyl Cyanogen mmol: ferrocene mmol: the ratio of tert-butyl hydroperoxide is 1: 2: 0.1: 2, and N-benzylaniline (91.6 mg, 0.5 mmol), ferrocene Iron (9.2 mg, 0.05 mmol), in the solvent dimethyl sulfoxide, under stirring, then add trimethylsilyl cyanide (125.1 μL, 1.0 mmol), tert-butyl hydroperoxide (148.0 μL, 1.0 mmol), After the addition, at 80°C, the catalytic oxidative cyanation reaction was carried out with continuous stirring for 4 hours.
[0029] In step (2), the eluent was a mixture of ethyl acetate:petroleum ether at a ratio of 1:50 to obtain α-cyano-N-benzylaniline (33.3 mg, yield 32%) as a white solid.
Embodiment 3
[0031] A kind of α-cyanation method of monoalkyl substituted aniline, its concrete steps are with embodiment 1, wherein:
[0032] (1) With N-benzylaniline as raw material, trimethylsilyl cyanide as cyanide reagent, ferrocene as catalyst, tert-butyl hydroperoxide as oxidant, according to N-benzylaniline mmol: trimethylsilyl Cyanogen mmol: ferrocene mmol: the ratio of tert-butyl hydroperoxide is 1: 2: 0.1: 2, and N-benzylaniline (91.6 mg, 0.5 mmol), ferrocene Iron (9.2 mg, 0.05 mmol), in the solvent dimethyl sulfoxide, under stirring, then add trimethylsilyl cyanide (125.1 μL, 1.0 mmol), tert-butyl hydroperoxide (148.0 μL, 1.0 mmol), After the addition is complete, the catalytic oxidative cyanation reaction is carried out at 35° C. for 4 hours with continuous stirring.
[0033] In step (2), the eluent was a mixture of ethyl acetate:petroleum ether at a ratio of 1:50 to obtain a white solid α-cyano-N-benzylaniline (53.1 mg, yield 51%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com