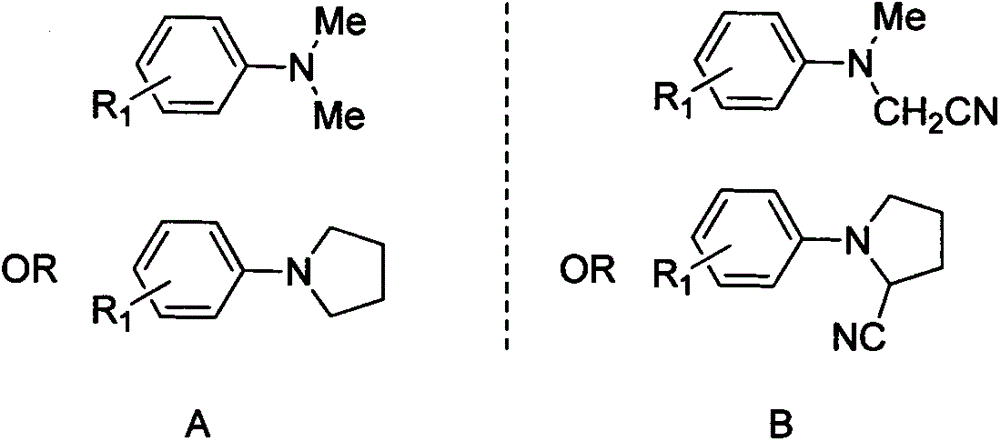
Method for N, N-dialkylaniline cyanation reaction
A technology of dialkylaniline and cyanation reaction, applied in the field of N, can solve the problems of increased production cost, large environmental pollution, and relatively expensive price, and achieve the effect of low production cost, small environmental pollution, and good market application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] A kind of N, the method for N-dialkylaniline cyanation reaction, its concrete steps are as follows:
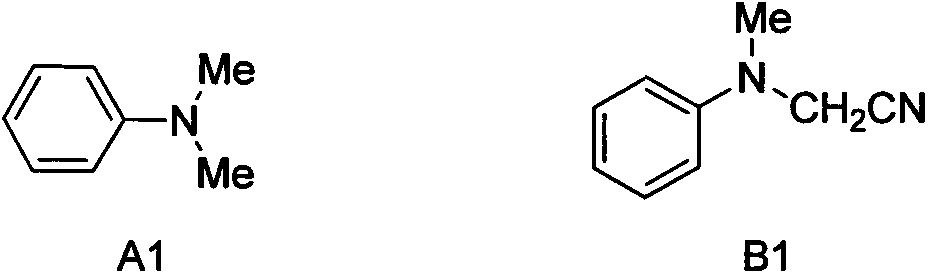
[0024] (1) With N,N-dimethylaniline A1 as raw material, bis(trifluoroacetoxy) iodobenzene as oxidizing agent, trimethylsilylcyanide as cyanating agent, anhydrous Na 2 SO 4 As an additive, 1,2-dichloroethane is a solvent, according to N, N-dimethylaniline A1 millimole: bis(trifluoroacetoxy) iodobenzene millimole: trimethylsilylcyanide millimole: additive without Water Na 2 SO 4 The ratio of mmoles: milliliters of solvent is the ratio of 1.0: 1.0: 2.0: 0.5: 6.0, and bis(trifluoroacetoxy) iodobenzene (215.0mg, 0.5mmol) and 1,2-dichloro Ethane (3.0mL), then added trimethylsilyl cyanide (99.2mg, 1.0mmol) under stirring, stirred for 40 minutes under ice bath, and then added anhydrous Na 2 SO 4 (36.0mg, 0.25mmol) and N, N-dimethylaniline A1 (61.0mg, 0.5mmol), after the addition is complete, continue stirring at this temperature for 1 hour to prepare N, N-dialkylaniline cy...
Embodiment 2
[0029] A kind of N, the method for N-dialkylaniline cyanation reaction, its concrete steps are with embodiment 1, wherein:
[0030] (1) With N,N-dimethylaniline A1 as raw material, bis(trifluoroacetoxy) iodobenzene as oxidizing agent, trimethylsilylcyanide as cyanating agent, anhydrous Na 2 SO 4 As an additive, 1,2-dichloroethane is a solvent, according to N, N-dimethylaniline A1 millimole: bis(trifluoroacetoxy) iodobenzene millimole: trimethylsilylcyanide millimole: additive without Water Na 2 SO 4 The ratio of millimoles: solvent milliliters is 1.0: 2.5: 2.0: 0.5: 6.0, and bis(trifluoroacetoxy) iodobenzene (538.0mg, 1.25mmol) and 1,2-dichloro Ethane (3.0mL), then added trimethylsilyl cyanide (99.2mg, 1.0mmol) under stirring, stirred for 40 minutes under ice bath, and then added anhydrous Na 2 SO 4 (36.0mg, 0.25mmol) and N, N-dimethylaniline A1 (61.0mg, 0.5mmol), after the addition is complete, continue stirring at this temperature for 1 hour to prepare N, N-dialkylanili...
Embodiment 3
[0033] A kind of N, the method for N-dialkylaniline cyanation reaction, its concrete steps are with embodiment 1, wherein:
[0034] (1) With N,N-dimethylaniline A1 as raw material, bis(trifluoroacetoxy) iodobenzene as oxidizing agent, trimethylsilylcyanide as cyanating agent, anhydrous Na 2 SO 4 As an additive, 1,2-dichloroethane is a solvent, according to N, N-two. Methylaniline A1 mmoles: two (trifluoroacetoxy) iodobenzene mmoles: trimethylsilylcyanide mmoles: additive Anhydrous Na 2 SO 4 The ratio of mmoles: milliliters of solvent is 1.0: 1.5: 1.5: 0.5: 6.0, and bis(trifluoroacetoxy) iodobenzene (323.0mg, 0.75mmol) and 1,2-dichloro Ethane (3.0mL), and trimethylsilylcyanide (75.0mg, 0.75mmol) was added under stirring, and anhydrous Na 2 SO 4 (36.0mg, 0.25mmol) and N, N-dimethylaniline A1 (61.0mg, 0.5mmol), after the addition is complete, continue stirring at this temperature for 1 hour to prepare N, N-dialkylaniline cyanide The reaction solution of compound B1.
[0035]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com