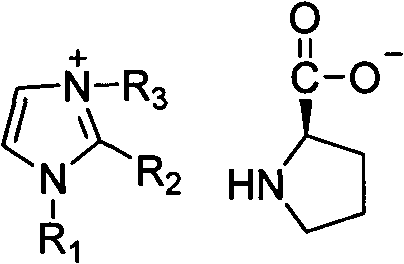
Alkyl imidazole-L-proline salt chiral ionic liquid and preparation method thereof
A chiral ionic liquid, proline salt technology, applied in the direction of organic chemistry, etc., can solve the problem of many reaction steps, and achieve the effect of good optical activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Example 1: 1,3-Dibutylimidazole-L-proline salt chiral ionic liquid
[0020] Raw materials: imidazole analytical reagent
[0021] Bromobutane Analytical grade reagent
[0022] L-Proline Analytical Reagent
[0023] Potassium hydroxide Analytical grade reagent.
[0024] DMSO Analytical Grade Reagents
[0025] Anhydrous ethanol analytical reagent
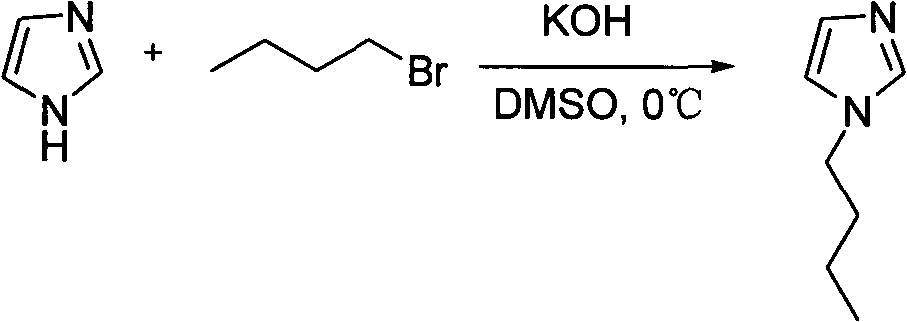
[0026] 1. Synthesis of N-butylimidazole
[0027] Add 4.136g (61mmol) of imidazole into a 100mL three-necked flask, add 4.099g (73mmol) of potassium hydroxide, add 30mL of DMSO, stir and dissolve, measure 73mmol of bromoalkane after 5h, add dropwise with a dropping funnel under ice bath, and react A large number of white solids appeared in the solution, and the reaction was stopped after stirring at room temperature for 11 h. The reaction solution was separated with distilled water and chloroform, and the chloroform layer was washed with (4×20 mL) distilled water, and the anhydrous MgSO 4 Drying overnight, filtering, and ro...
Embodiment 2
[0043] Example 2: 1,2-Dimethyl-3-butylimidazole-L-proline salt chiral ionic liquid
[0044] Raw material: 1,2-Dimethylimidazole Analytical reagent
[0045] Bromobutane Analytical grade reagent
[0046] L-Proline Analytical Reagent
[0047] Potassium hydroxide Analytical grade reagent.
[0048] DMSO Analytical Grade Reagents
[0049] Absolute ethanol Analytical grade reagent
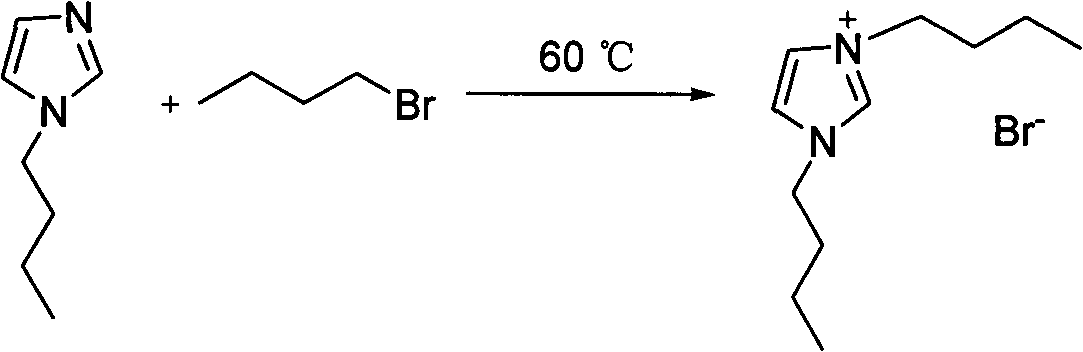
[0050] 1. Synthesis of 1,2-dimethyl-3-butylimidazole bromide
[0051]
[0052] 1,2-Dimethylimidazole N-Butane Bromide 1,2-Dimethyl-3-Butylimidazole Bromide
[0053] Add 45mmol of 1,2-dimethylimidazole and 50mmol of n-butane bromide into a 100mL three-necked flask. The preparation method is the same as that of 1,2-dibutylimidazole bromide in Example 1.
[0054] 2. Synthesis of 1,2-dimethyl-3-butylimidazolium proline salt chiral ionic liquid
[0055]
[0056] 1,2-Dimethyl L-proline bromide 1,2-Dimethyl-3-butyl
[0057] -3-Butylimidazole imidazole-L-proline salt
[0058] In a 100mL three-necke...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical rotation | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com