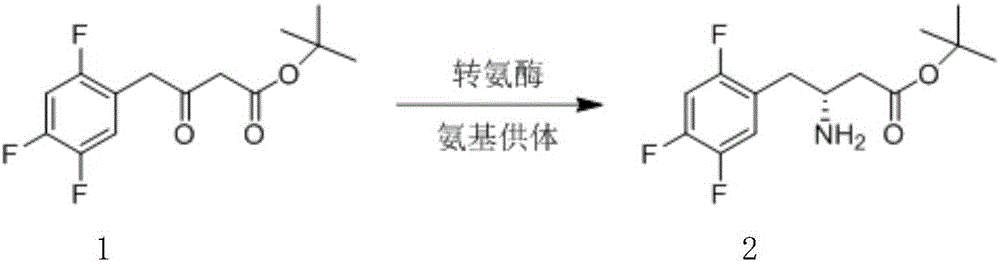
Biological preparation method of (R)-3-amino-4-(2,4,5-trifluorophenyl) tert-butyl butyrate
A trifluorophenyl, biological preparation technology, applied in the field of biopharmaceuticals and green chemistry, can solve the problems of residual protein risk, high separation cost, poor stability, etc., and achieve no protein residue, high optical activity, simple and convenient operation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] In a 20mL three-neck flask, add 3.2mL of 0.1M pH 9.5 triethanolamine-hydrochloric acid buffer solution, 500mg of substrate, 900μL of isopropylamine, 0.4mL of Tween 60, 0.4mL of DMSO, 5mg of pyridoxal phosphate (PLP), transaminase EW1101 (Suzhou Hanzyme Biotechnology Co., Ltd.) 50mg, at 30°C, 200rpm paddle stirring, 0.01MPa nitrogen purging, dropwise added 4M isopropylamine to control the pH at 9.5, reacted for 24h, and the conversion rate was 95% by HPLC. Add hydrochloric acid to adjust the pH to 2-3, filter with diatomaceous earth, add an equal volume of ethyl acetate to extract twice, and rotary evaporate to obtain 90 mg of the product with a purity of 98% and an optical purity of >99%.
Embodiment 2
[0023] In the reactor, 3.2L of 0.1M pH 9.5 triethanolamine-hydrochloric acid buffer solution, 500g of substrate, 900mL of isopropylamine, 0.4L of Tween 60, 0.4mL of DMSO, 5g of pyridoxal phosphate (PLP), transaminase EW1101 ( Suzhou Hanzyme Biotechnology Co., Ltd.) 50g, at 30°C, under the conditions of mechanical stirring and 0.01MPa nitrogen purge, add 4M isopropylamine dropwise to control the pH at 9.5, react for 24h, and the conversion rate by HPLC is 95%. Add hydrochloric acid to adjust the pH to 2-3, filter with diatomaceous earth, add an equal volume of ethyl acetate to extract twice, and rotary evaporate to obtain 90 g of the product with a purity of 98% and an optical purity of >99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com