Application of carbonyl acyl reductase in preparing (S)-4-chlorine-3 hydroxyl ethyl butyrate
A technology of ethyl hydroxybutyrate and carbonyl reductase, which is applied in the biological field to achieve the effects of reduced production costs, high yield, and high optical activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1: Acquisition of carbonyl reductase gene
[0031] Pichia Stipitis CBS 6054 (purchased from Centraalbureau voor Schimmelcultures (CBS) Fungal Biodiversiry Centre), medium YPD (g L -1 ): Yeast extract 10g, peptone 20g, glucose 20g, add distilled water to 1L.
[0032] Pichia Stipitis CBS 6054 was inoculated in 5mL LYPD liquid medium and cultured at 30°C to logarithmic growth phase, and the genome was extracted using a genomic DNA extraction kit (Beijing Tianwei Bioengineering Co., Ltd. Yeast Genome Extraction Kit).
[0033] The primers used to construct the expression vectors are provided with enzyme cutting sites, and the primer sequences are as follows:
[0034] The upstream primer (CRII-sense containing Nde I) is:
[0035] 5'-GGAATTC CATATG ACTGTCGAAACCGCCACC-3'
[0036] Downstream primers (CRII-anti containing BamH I) are:
[0037] 5'-CGC GGATCC CTAGACAGAACAGTAACCACCT-3'
[0038] All primers were synthesized by Shanghai Shenergy Gaming Company.
[00...
Embodiment 2
[0041] Example 2: Expression of Genes
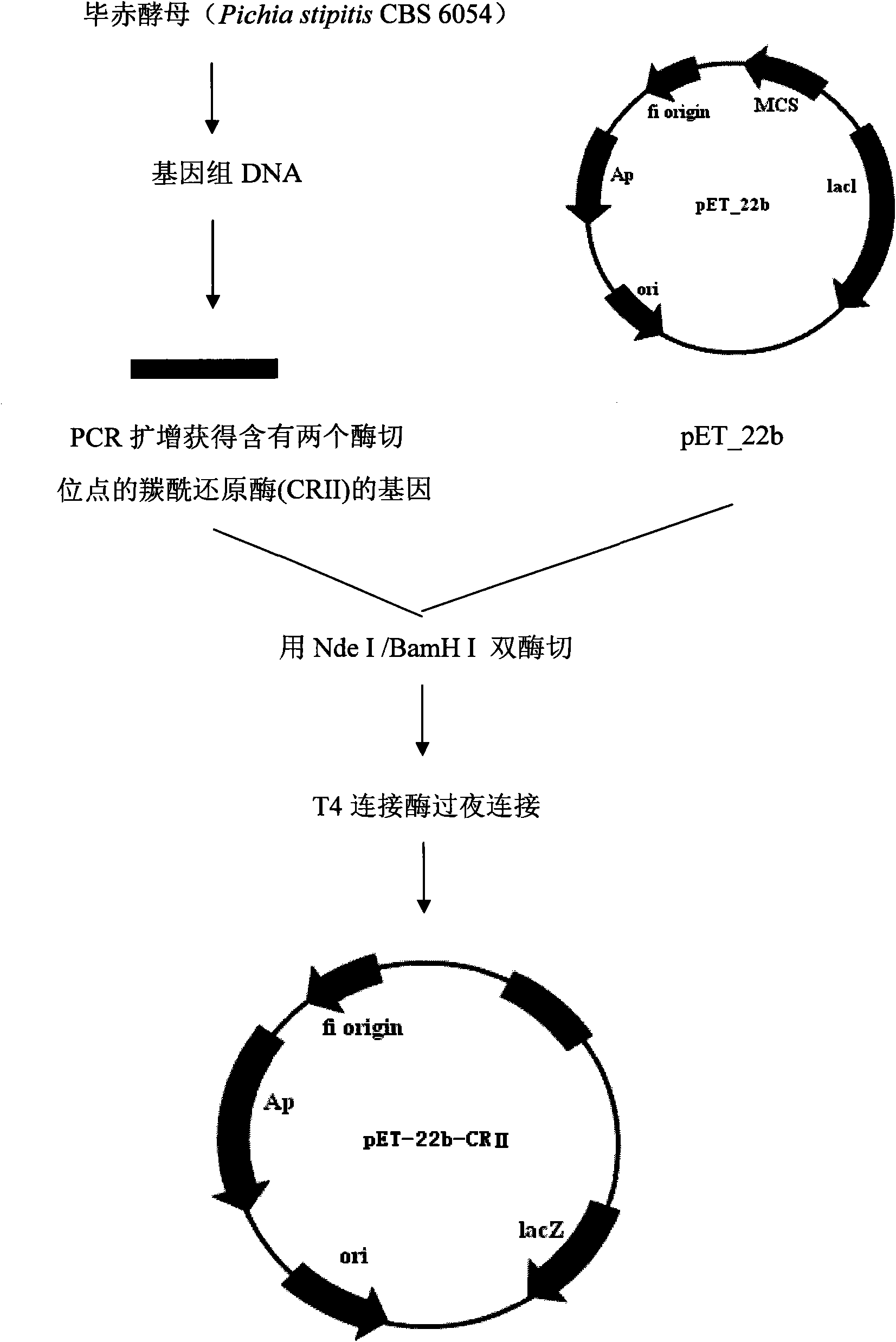
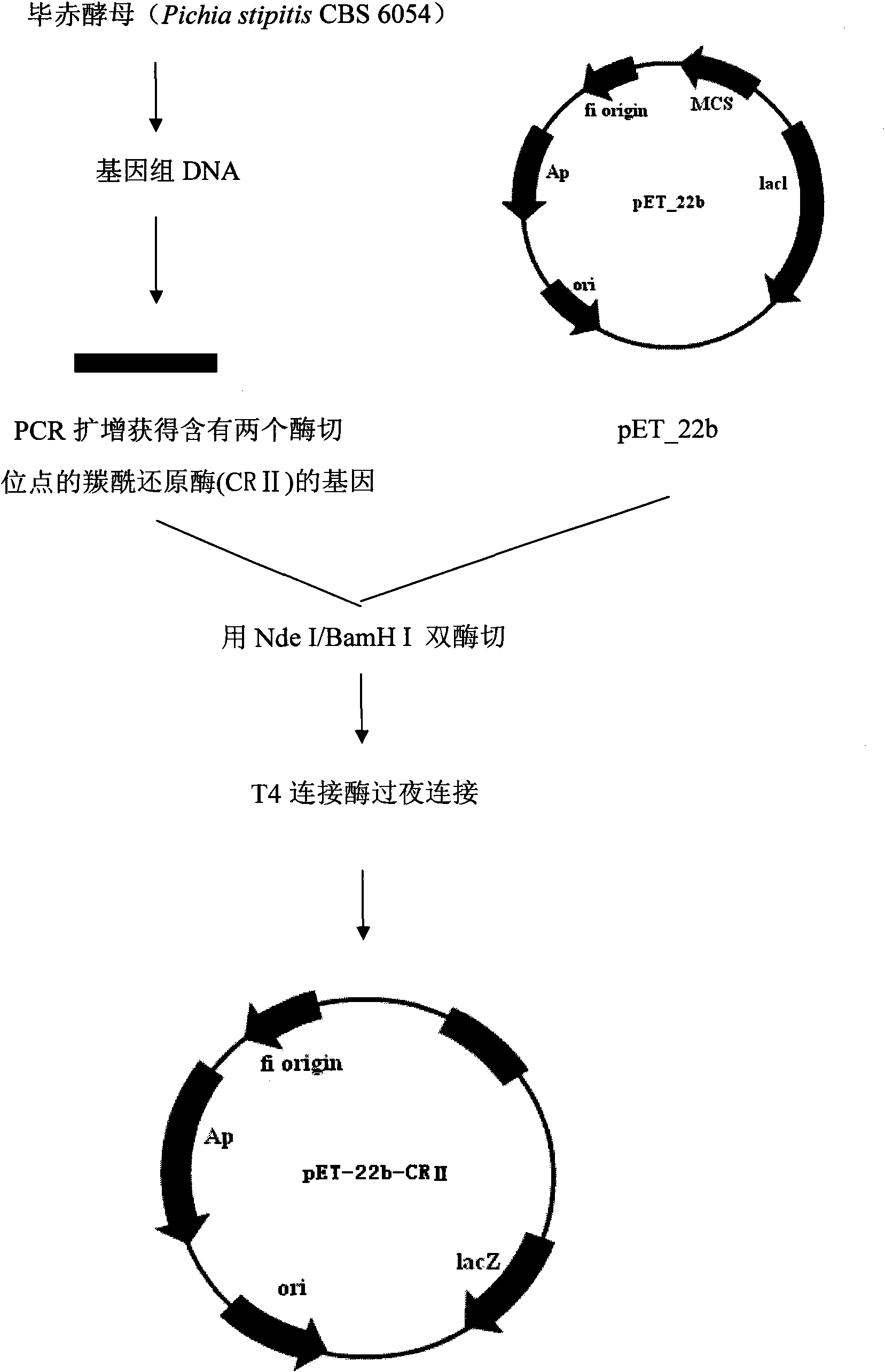
[0042] Use Nde I and BamH I to respectively digest pET-22b (pET-22b was purchased from Novagen (Merck China)) and the amplified target gene containing two restriction sites, and recover the double-digested target fragments by gel respectively and the expression vector, the double-digested expression vector pET-22b and the target gene were ligated overnight with T4 ligase, and 10uL of the ligation product pET-22b-PsCRII was added to 100uL of Rosetta (DE3) competent cells, on ice Leave it for 30min, heat shock at 42°C for 90sec. Place on ice for 2 minutes. Add 0.45 mL of pre-warmed medium. 220rpm 37°C for 1h. Add 200 uL of bacterial solution to LB plates containing 100 μg / mL ampicillin and chloramphenicol respectively, and culture overnight at 37°C for 12-16 hours. To build a graph see figure 1 .
Embodiment 3
[0043] Embodiment 3: the mensuration of enzyme activity
[0044] Pick the recombinant bacteria E.coli Rosseta (pET-22b-PsCR) and Escherichia coli Rosseta (DE3) into the LB liquid medium containing antibiotics, and cultivate overnight at 37°C with shaking. Then inoculate into fresh culture medium according to 2% inoculum amount, and cultivate to OD at 37°C 600 At about 0.6, add IPTG to a final concentration of 0.8mmol·L -1 , 25°C, 220rpm, after induction of expression for 10h, centrifuge (4°C, 5000rpm, 15min), resuspend the bacteria sludge with 100mM potassium phosphate buffer (pH7.0), and sonicate the cells (power 300W, ultrasonic 5s, intermittent 5s, total 5min), centrifuge (4°C, 12000rpm, 15min), and measure the enzyme activity in the supernatant.
[0045] The enzyme reaction system includes 100mM potassium phosphate buffer (pH6.0), 5mM NADH, 20mM COBE, 30°C, and the decrease of absorbance value at 340nm is measured. Enzyme activity is defined as the amount of enzyme requ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com