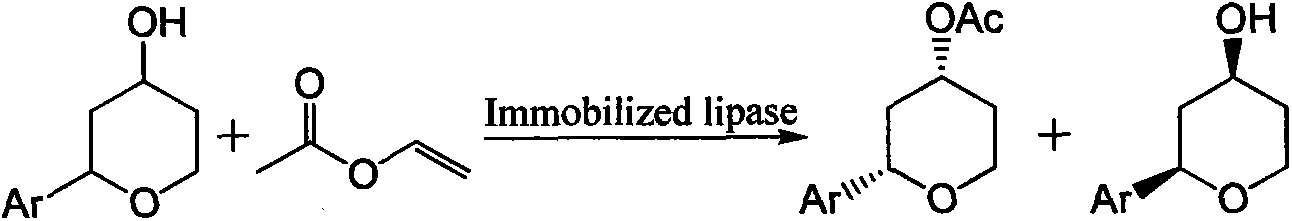Method for preparing 4-hydroxyl tetrahydropyran derivative with optical activity through enzymatic transesterification
A technology of hydroxytetrahydropyran derivatives and optical activity, which is applied in the field of preparation of magnetic inorganic-organic complex immobilized enzymes as catalysts, can solve the problems of easy clustering deactivation, mechanical loss, poor stability, etc., and achieve The effect of high reaction enantioselectivity, no mechanical loss, and high stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030]Example 1 Preparation of Optically Active 2-Phenyl-4-Hydroxytetrahydropyran and Its Acetate by Enzyme-Catalyzed Transesterification
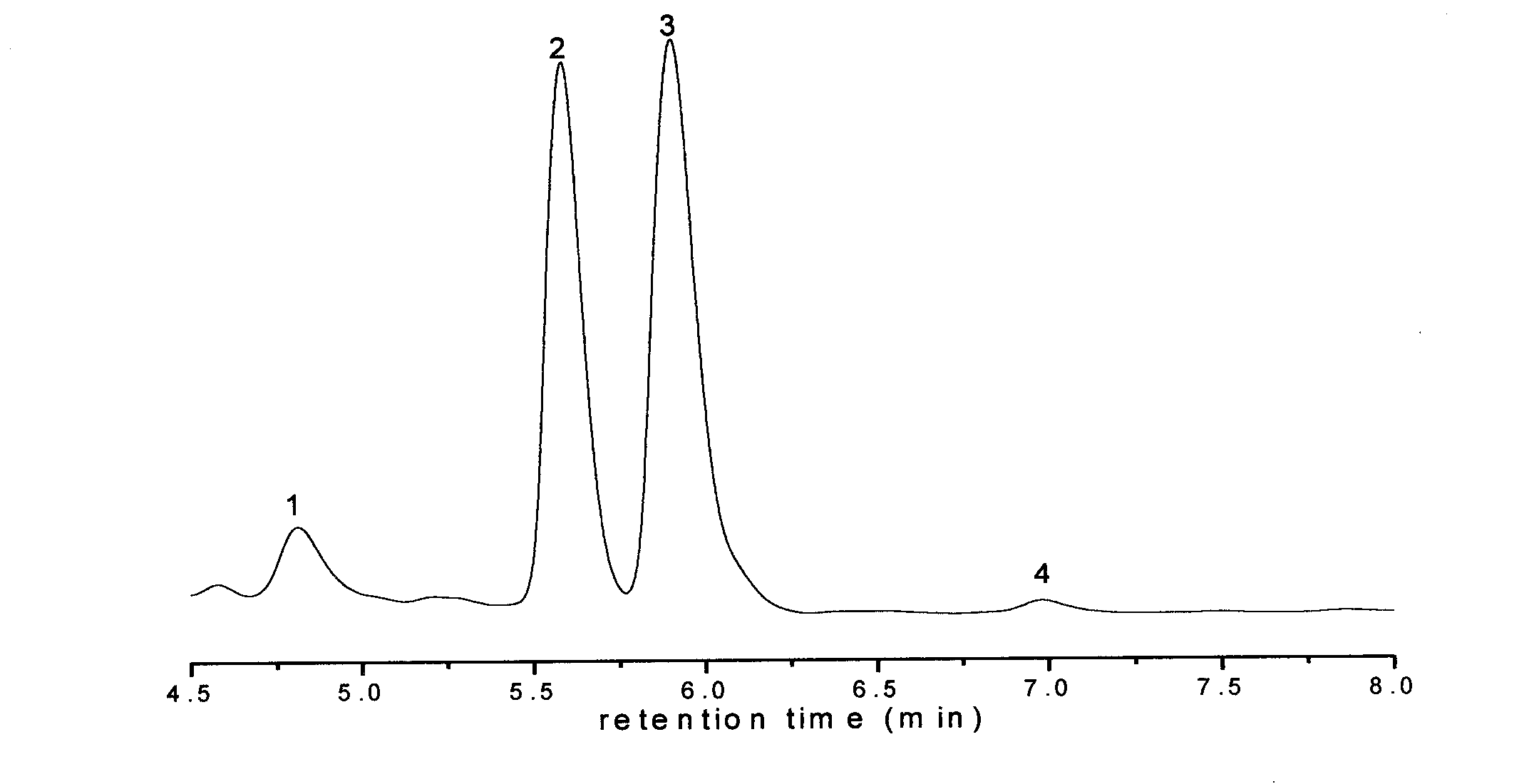
[0031] Add 0.05mol of 2-benzene-4-hydroxytetrahydropyran and 0.05mol of vinyl acetate into a 250ml reactor, then add 100ml of heptane and mix well, then add 0.1g of magnetic Fe 3 o 4 SiO 2 -(CH 2 ) 3 -NH 2 Immobilized PSL enzyme, reacted under stirring at 30°C for 24 hours, separated the immobilized enzyme in a magnetic field, and obtained the enantiomer of the chiral enantiomer (2R, 4S)-2-benzene-4-hydroxytetrahydropyran body excess value ee s 99.0%, enantiomeric excess ee of (2S,4R)-2-phenyltetrahydropyran-4-ol acetate p was 88.6%.
Embodiment 2
[0032] Example 2 Preparation of Optically Active 2-Phenyl-4-Hydroxytetrahydropyran and Its Acetate by Enzyme-Catalyzed Transesterification
[0033] Add 0.05mol of 2-benzene-4-hydroxytetrahydropyran and 0.05mol of isopropenyl acetate into a 250ml reactor, then add 100ml of cyclohexane and mix well, then add 0.1g of magnetic MnFe 2 o 4 SiO 2 -(CH 2 ) 3 -NH 2 Immobilized PSL enzyme, reacted for 36 hours under stirring at 30°C, separated the immobilized enzyme in a magnetic field, and the content of the obtained chiral enantiomer was analyzed by chiral column high performance liquid chromatography, (2R, 4S)-2- Enantiomeric Excess Value ee of Benzene-4-Hydroxytetrahydropyran s 98.0%, enantiomeric excess value ee of (2S,4R)-2-phenyltetrahydropyran-4-ol acetate p was 87.6%.
Embodiment 3
[0034] Example 3 Preparation of Optically Active 2-Benzene-4-Hydroxytetrahydropyran and Its Acetate by Enzyme-Catalyzed Transesterification
[0035] Add 0.05mol of 2-benzene-4-hydroxytetrahydropyran and 0.05mol of vinyl acetate into a 250ml reactor, then add 100ml of toluene and mix well, then add 0.1g of magnetic MnFe 2 o 4 SiO 2 -(CH 2 ) 3 -NH 2 Immobilized CRL enzyme, reacted under stirring at 30°C for 24 hours, separated the immobilized enzyme in a magnetic field, and obtained the enantiomer of (2R, 4S)-2-benzene-4-hydroxytetrahydropyran body excess value ee s 99.0%, enantiomeric excess value ee of (2S,4R)-2-phenyltetrahydropyran-4-ol acetate p was 92.0%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com