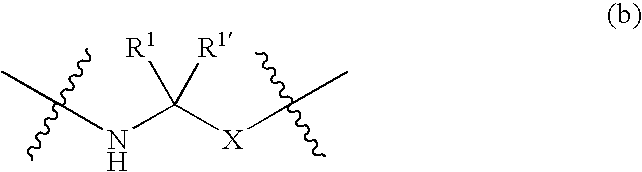
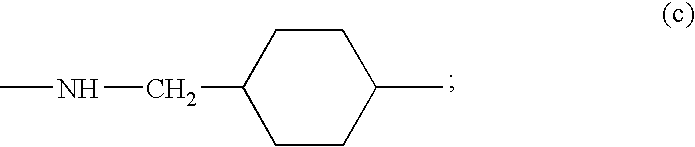
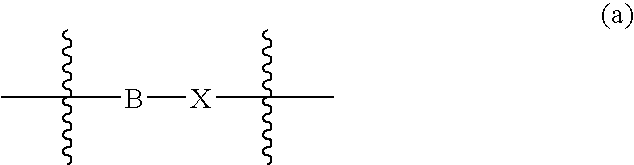Somatostatin antagonists and agonists that act at the sst subtype 2 receptor
a subtype 2 receptor and antagonist technology, applied in the field of pharmaceutical active compounds, can solve the problems of short half-life of growth hormone in the body, difficult in practice, and permanent short statur
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
6-Amino-2-[2-[(biphenyl-4-ylmethyl)-amino]-3-(1H-indol-3-yl)-propionylamino]-hexanoic acid methyl ester, Having the Indicated Stereospecificity.
Step 1
Wang resin (Arogel Wang, Argonaut Technologies, 170 mg, 0.39 mmol / g. 0.066 mmol) was allowed to swell in 3 mL CH2Cl2 for 15 min, and washed 3× (3 times) with 3 mL CH2Cl2. A solution of Fmoc-Lsy(Boc)-OH (128 mg, 0.26 mmol), DIC (38 uL, 0.26 mmol), TEA (70 uL, 0.5 mmol) and DMAP (3 mg, 0.026 mmol) in 2.5 mL CH2Cl2 was added, and the mixture was agitated by rotation for 1.5 h. The resin was then washed, consecutively, 3× with 3 mL CH2Cl2, 2× with 3 mL DMF, 2× with 3 mL EtOH, and finally 3× with 3 mL CH2Cl2. 3 mL of a 20% solution of piperidine in CH2Cl2 was then added, and the composition was agitated by rotation for 1 h. The resin was then washed, consecutively, 3× with 3 mL CH2Cl2, 2× with 3 mL DMF, 2× with 3 mL EtOH, and 3× with 3 mL CH2Cl2. A solution of Fmoc-d-Trp-OH (110 mg, 0.26 mmol),), DIC (38 uL, 0.26 mmol), TEA (70 uL, 0....
example 2
2-{3-(3-Fluoro-phenyl)-2-[2-(toluene-4-sulfonylamino)-acetylamino]-propionylamino}-5-guanidino-pentanoic acid methyl ester, Having the Indicated Stereospecificity.
Step 1 Preparation of 3-F-Phe-Arg(NO2)—OMe
To a solution of 2.00 gm of BOC-3-F-Phe-OH (7.06 mmol), 2.09 gm of Arg(NO2)—OMe HCl (7.77 mmol), 1.05 gm of hydroxybenzotriazole and 2.58 gm of 4-dimethylaminopyridine in 50 mL of methylene chloride was added 1.5 gm of 1,3-dimethylaminopropyl-3-ethylcarbodiimide hydrochloride. After stirring for 15 hours 100 mL more methylene chloride was added to the reaction, and it was washed three times with 100 mL portions of 10% aqueous hydrochloric acid solution, twice with 100 mL of 50% saturated sodium bicarbonate solution, once with 100 mL of saturated brine, dried over anhydrous magnesium sulfate, filtered, and the solvent removed under reduced pressure to yield 2.96 gm of product. This was dissolved in 100 mL of 10% trifluoroacetic acid in methylene chloride, stirred for 2 hours, ...
example 3
6-Amino-2-[2-[(biphenyl-4-carbonyl)-amino]-3-(1H-indol-3-yl)-propionylamino]-hexanoic acid methyl ester, Having the Indicated Stereospecificity.
1H NMR (CD3OD): δ 4.32 (m, 1H); 3.39 (d, 1H); 3.63 (s, 3H); 2.87 (m, 2H). MS: M+1=527
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| circulatory concentrations | aaaaa | aaaaa |
| affinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com