A kind of varenicline tartrate tablet and preparation method thereof
A technology for varenicline tartrate and tablets, which is applied in the directions of pill delivery, pharmaceutical formulations, and non-active ingredients medical preparations, etc., can solve the problems of reduced dissolution rate of tablets, unsuitable for long-term use, cumbersome process, etc. High dissolution, low friability and good fluidity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
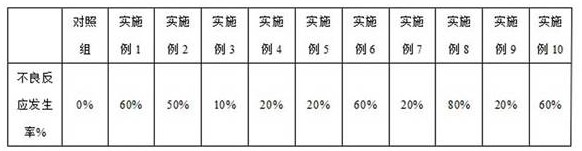
Examples
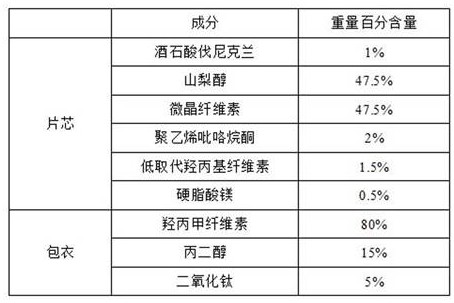
Embodiment 1
[0051]
[0052] 1) when one third of the weight of magnesium stearate, microcrystalline cellulose and varenicline tartrate are mixed after 20 minutes at a rotating speed of 15rpm, sieve and granulate, wherein the screen aperture is 0.8-2.0mm during granulation, to prepare a mixture;
[0053] 2) mixing the mixture obtained in step 1) with mannitol, polyvinylpyrrolidone, and low-substituted hydroxypropyl cellulose for 10-30 minutes at a rotating speed of 15 rpm;
[0054] 3) adding residual magnesium stearate to the mixture obtained in step 2), and mixing for 10-20 minutes at a rotating speed of 15 rpm;
[0055] 4) compressing the obtained mixed granules in step 3), and controlling the hardness of the tablet to be 3~10Kp;
[0056] 5) Transfer the plain tablet compressed in step 4) to a coating machine for coating operation.
Embodiment 2
[0058]
[0059] 1) when one third of the weight of magnesium stearate, microcrystalline cellulose and varenicline tartrate are mixed after 20 minutes at a rotating speed of 15rpm, sieve and granulate, wherein the screen aperture is 0.8-2.0mm during granulation, to prepare a mixture;
[0060] 2) mixing the obtained mixture of step 1) with calcium hydrogen phosphate, polyvinylpyrrolidone, and low-substituted hydroxypropyl cellulose for 10-30 minutes at a rotating speed of 15 rpm;
[0061] 3) adding residual magnesium stearate to the mixture obtained in step 2), and mixing for 10-20 minutes at a rotating speed of 15 rpm;
[0062] 4) compressing the obtained mixed granules in step 3), and controlling the hardness of the tablet to be 3~10Kp;
[0063] 5) Transfer the plain tablet compressed in step 4) to a coating machine for coating operation.
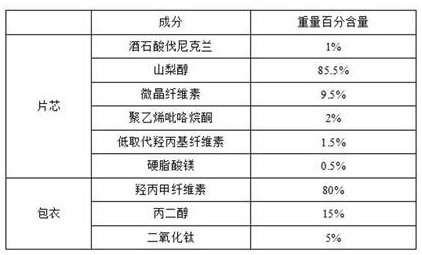
Embodiment 3
[0065]
[0066] 1) when one third of the weight of magnesium stearate, microcrystalline cellulose and varenicline tartrate are mixed after 20 minutes at a rotating speed of 15rpm, sieve and granulate, wherein the screen aperture is 0.8-2.0mm during granulation, to prepare a mixture;
[0067] 2) mixing the mixture obtained in step 1) with sorbitol, polyvinylpyrrolidone, and low-substituted hydroxypropyl cellulose for 10-30 minutes at a rotating speed of 15 rpm;
[0068] 3) adding residual magnesium stearate to the mixture obtained in step 2), and mixing for 10-20 minutes at a rotating speed of 15 rpm;
[0069] 4) compressing the obtained mixed granules in step 3), and controlling the hardness of the tablet to be 3~10Kp;
[0070] 5) Transfer the plain tablet compressed in step 4) to a coating machine for coating operation.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com