Varenicline pharmaceutical composition capable of reducing generation of nitrosamine impurities, and preparation and application of varenicline pharmaceutical composition
A technology of varenicline and its composition, which is applied in the field of varenicline pharmaceutical composition, can solve problems such as excessive content of nitrosamine impurities, achieve the effects of reducing the generation of nitrosamine impurities, ensuring drug safety, and improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
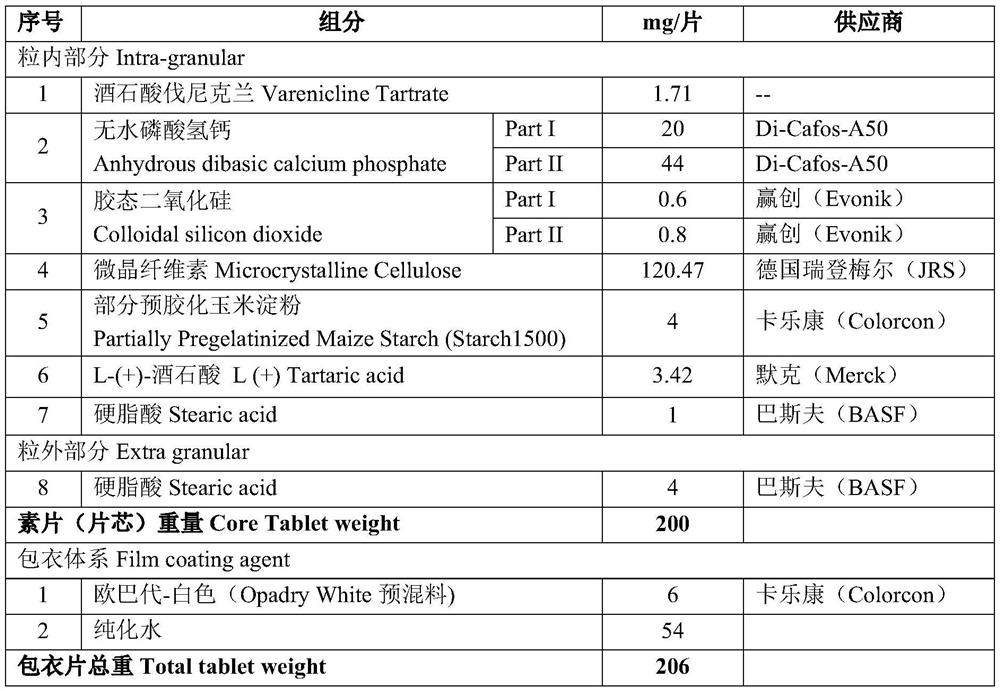
[0163] Table 1. Components and contents of formula A (specification 1 mg)
[0164]
[0165] in,
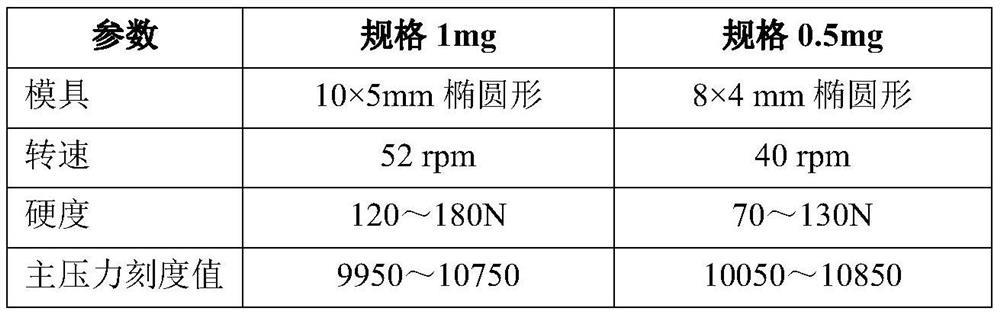
[0166] 1.71mg / tablet of varenicline tartrate can be converted into 1mg / tablet of varenicline, that is, each tablet contains 1mg of active ingredient (varenicline), which can also be expressed as: 1mg plain tablet (or 1mg coated tablet, etc.) . And the 0.5mg tablet is that the amount of each component is reduced to half on the basis of the 1mg tablet. In the above formulation, microcrystalline cellulose and anhydrous calcium hydrogen phosphate were used as diluents, partially pregelatinized corn starch was used as a disintegrant, colloidal silicon dioxide was used as a glidant, and stearic acid was used as a lubricant , L-(+)-tartaric acid is used as an acidity regulator (or called a pH regulator), and purified water is used as a coating solvent.
[0167] The preparation method (every batch of 130,000 pieces) comprises the following steps:
[0168] ⑴ Screening and mixing
...
Embodiment 2
[0187] Formula B (specification 1 mg): change the dosage of L-(+)-tartaric acid to 1.71 mg / tablet, and keep other conditions unchanged. According to the same preparation method as in Example 1, 1 mg plain tablet B, 1 mg white coated tablet B and 1 mg blue coated tablet B can be prepared.
Embodiment 3
[0189] Formula Y (specification 1mg): L-(+)-tartaric acid is not added to the tablet, and other conditions remain unchanged. According to the same preparation method as in Example 1, 1 mg plain tablet Y, 1 mg white coated tablet Y and 1 mg blue coated tablet Y can be prepared.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| absorption wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com