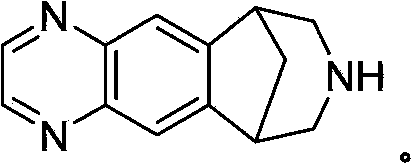
Preparation method for varenicline intermediate
A technology of varenicline and intermediates, applied in the directions of oxidation reaction preparation and the like, can solve the problems of low yield, difficult product purification, unsuitable for industrial amplification, etc., and achieves simple synthesis steps, mild reaction conditions, and low production costs. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
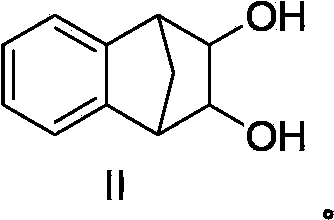
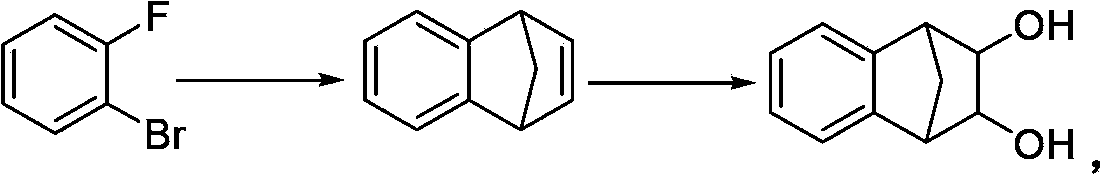
[0033] Add 50g (352mmol, 1.0eq) of 1,4-dihydro-1,4-methanone, 123.6g (1.05mol, 3.0eq) of N-methyl-N-morpholine oxide, 62mg ( 0.18mmol, 0.05%eq) Potassium osmate dihydrate, 250mL methanol, 250mL water, the system was clear after stirring, and stirred at 20~30°C for 8 hours. HPLC detected that the reaction of the raw materials was complete. The reaction system was concentrated under reduced pressure to evaporate methanol, and water was added to 250 mL. After stirring, the mixture was filtered, and the filter cake was washed with 50 mL of petroleum ether. After the filter cake was dried, 53 g of powdery solid was obtained, which was the varenicline intermediate (compound II), with a melting point of 176-178°C, a content of 98%, a purity of 99.7%, and a yield of 83.8%.
Embodiment 2
[0035] Add 50g (352mmol, 1.0eq) 1,4-dihydro-1,4-methyconaphthalene, 123.6g (1.05mol, 3.0eq) N-methyl-N-morpholine oxide, 62mg ( 0.18mmol, 0.05%eq) Potassium osmate dihydrate, 350mL methanol, 150mL water, the system was clear after stirring, and stirred at 10~20°C for 15 hours. HPLC detected that the reaction of the raw materials was complete. The reaction system was concentrated under reduced pressure to evaporate methanol, and water was added to 250 mL. After stirring, the mixture was filtered, and the filter cake was washed with 50 mL of petroleum ether. After the filter cake was dried, 52 g of powdery solid was obtained, which was the varenicline intermediate (compound II), with a melting point of 176-178°C, a content of 99%, a purity of 99.5%, and a yield of 83.1%.
Embodiment 3
[0037] Add 50g (352mmol, 1.0eq) 1,4-dihydro-1,4-methyconaphthalene, 123.6g (1.05mol, 3.0eq) N-methyl-N-morpholine oxide, 62mg ( 0.18mmol, 0.05%eq) Potassium osmate dihydrate, 50mL methanol, 450mL water, the system was clear after stirring, and stirred at 40~50°C for 30 hours. HPLC detected that the reaction of the raw materials was complete. The reaction system was concentrated under reduced pressure to evaporate methanol, and water was added to 250 mL. After stirring, the mixture was filtered, and the filter cake was washed with 50 mL of petroleum ether. After the filter cake was dried, 46 g of powdery solid was obtained, which was the varenicline intermediate (compound II), with a melting point of 176-178°C, a content of 98%, a purity of 99.0%, and a yield of 72.7%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com