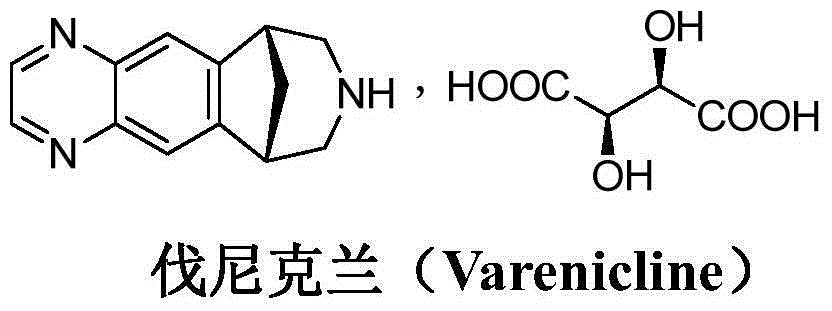
Preparation method of varenicline intermediate and nitroreduction impurity thereof
A technology for varenicline and intermediates, which is applied in the field of preparation of varenicline intermediates and nitro-reduction impurities, can solve the problems of difficult control of hydrogen sulfide equivalent, incomplete reaction, etc., and achieve reduced purification operations and good selectivity The effect of reduction and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
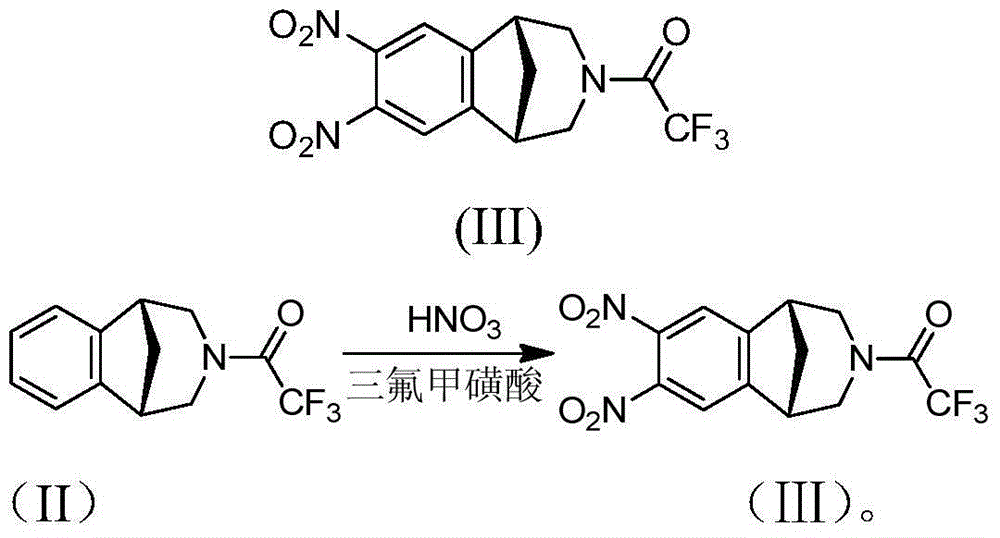
[0019] Embodiment 1: the preparation of varenicline intermediate
[0020] Add trifluoromethanesulfonic acid (1.23g) and dichloromethane (10ml) into the reaction flask, add concentrated nitric acid (0.25g) dropwise and stir, add compound (II) (1g) dropwise at -50~-60°C and react at room temperature After 6 hours, TLC monitored the completion of the reaction, added water, extracted three times with dichloromethane, washed the organic phase with water, dried, and evaporated to dryness to obtain compound (Ⅲ) (1g); Ester = 1:1, Rf = 0.3), and the purity of the product was 97% as detected by HPLC.
Embodiment 2
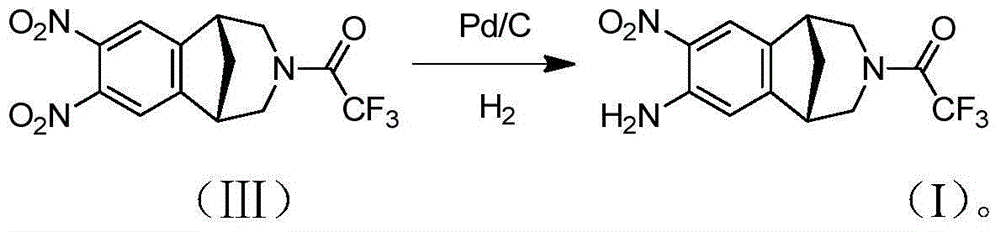
[0021] Embodiment 2: the preparation of selective nitro reduction impurity
[0022] Compound (Ⅲ) (1g), methanol (10mL) and Pd / C (0.1g) (60% water content) were added to the reaction flask, replaced by hydrogen three times, reacted at room temperature, TLC monitored the completion of the reaction, filtered, and evaporated to dryness to obtain Compound (I); off-white solid, yield 90.5%. The TLC spot plate is a single spot, under TLC conditions (developing agent n-hexane: ethyl acetate = 1:1, Rf = 0.2), and the purity of the product is 98% as detected by HPLC. MS-ESI(m / z):[M+H] + 316.22.
Embodiment 3
[0024] Add compound (III) (1g), methanol (10mL) and Pd / C (0.1g) (50% water content) into the reaction flask, replace with hydrogen three times, react at room temperature, monitor the completion of the reaction by TLC, filter and evaporate to dryness to obtain Compound (I); off-white solid, yield 85.5%. The TLC spot plate is a single point, under TLC conditions (developing agent n-hexane: ethyl acetate = 1:1, Rf = 0.2), and the purity of the product is 93% as detected by HPLC. MS-ESI(m / z):[M+H] + 316.22.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com