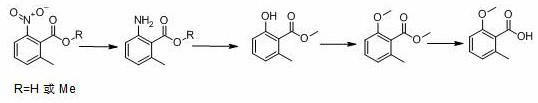
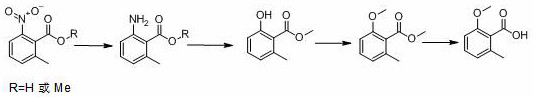
Preparation method of 2-methoxy-6-methylbenzoic acid
A kind of technology of methyl benzoic acid and methyl benzoate is applied in the field of preparation of 2-methoxy-6-methyl benzoic acid, and can solve the difficulty of 2-methoxy-6-methyl benzoic acid source , the lack of reports, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] (1) Reductive hydrogenation
[0035] Add 2-methyl-6-nitrobenzoic acid (500 g), platinum carbon (platinum content: 2%, 50 g), methanol (2000 g), into the autoclave, replace with nitrogen, and pass hydrogen to a pressure of 1.0-1.3 MPa, raise the temperature to 70-80°C, control the temperature and control the reaction pressure until the reaction is complete (raw material <0.5%), continue to stir for 60 min, filter while hot, collect the catalyst for mechanical use, and directly carry out diazotization hydrolysis of the mother liquor.
[0036] (2) Diazotization hydrolysis
[0037] The temperature of the reduction mother liquor obtained in step (1) is lowered to 0-5°C, and 900 g of nitrosyl sulfuric acid (weight percentage: 40%, 1.02 eq.) is added dropwise under temperature control. 30min, after stirring for 1 hour, heat up to 64-66°C and reflux for 8-12 hours until the reaction is complete (HPLC: 2-hydroxy-6-methylbenzoic acid+2-methoxy-6-methylbenzoic acid<1 %).
[003...
Embodiment 2
[0046] (1) Reductive hydrogenation
[0047] Add 2-methyl-6-nitrobenzoic acid (450 g), platinum carbon (platinum content: 2%, 9.0 g), methanol (1350 g), into the autoclave, nitrogen replacement, and hydrogen pressure to 0.9-1.1 MPa, raise the temperature to 60-70°C, control the temperature and control the reaction pressure until the reaction is complete (raw material <0.5%), continue to stir for 30 min, filter while hot, divide the mother liquor into three parts while hot, and directly carry out diazotization hydrolysis.
[0048] (2) Diazotization hydrolysis
[0049] The reduction mother liquor (1 / 3 amount) obtained in step (1) is cooled to 5-10°C, and 317.3 g of nitrosyl sulfuric acid (mass concentration 40%, 1.2 eq.) is added dropwise under temperature control. After the addition is completed, the temperature is slowly raised to 50-60 ℃, control the heating time not less than 30min, after stirring for 1 hour, raise the temperature to 64-66℃ and reflux for 12-16 hours until t...
Embodiment 3
[0055] diazotization hydrolysis
[0056] The reduction mother liquor (1 / 3 amount) obtained in step (1) of Example 2 was cooled to 10-15°C, and 277.6 g of sodium nitrite solution (mass concentration 40%, 1.05 eq.) was added dropwise under temperature control, and the addition was completed slowly Raise the temperature to 50-60°C, stir for 1 hour, then raise the temperature to 64-66°C and reflux for 8-16 hours until the reaction is complete (HPLC: 2-hydroxy-6-methylbenzoic acid + 2-methoxy-6-methyl benzoic acid <1%).
[0057] Raise the temperature to 85-100°C, recover about 360 g of methanol by atmospheric distillation, add 400 g of water, let stand to separate layers, and separate 105 g of oil layer.
[0058] The aqueous layer was extracted with MIBK 100ml, the organic layer was separated and concentrated, and then combined with the oil layer to obtain 141 g of crude product of methyl 2-hydroxy-6-methylbenzoate (HPLC: methyl 2-hydroxy-6-methylbenzoate: 45.6%; 2-methoxy-6-meth...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com