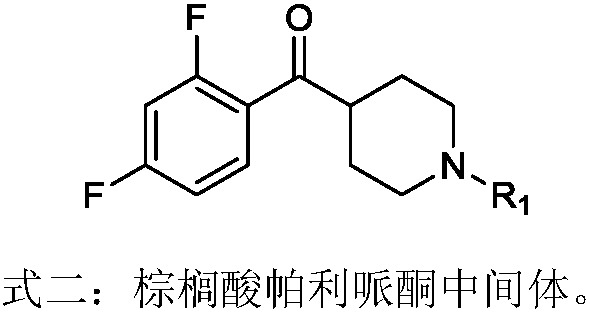
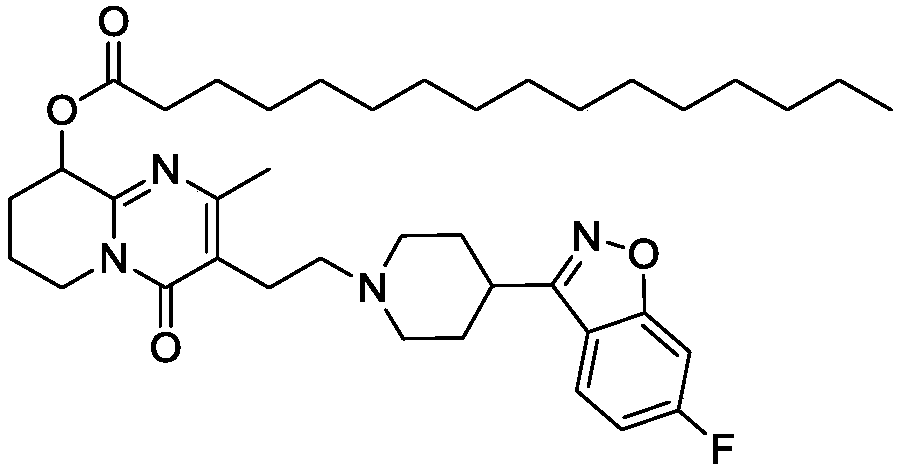
Preparation method of palmitic acid paliperidone intermediate
A technology for palmitic acid and intermediates, applied in the field of preparation of paliperidone palmitic acid intermediates, can solve the problems of low total yield and high cost of raw materials, and achieve the advantages of reducing impurity content, reducing purification operations and saving production costs. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
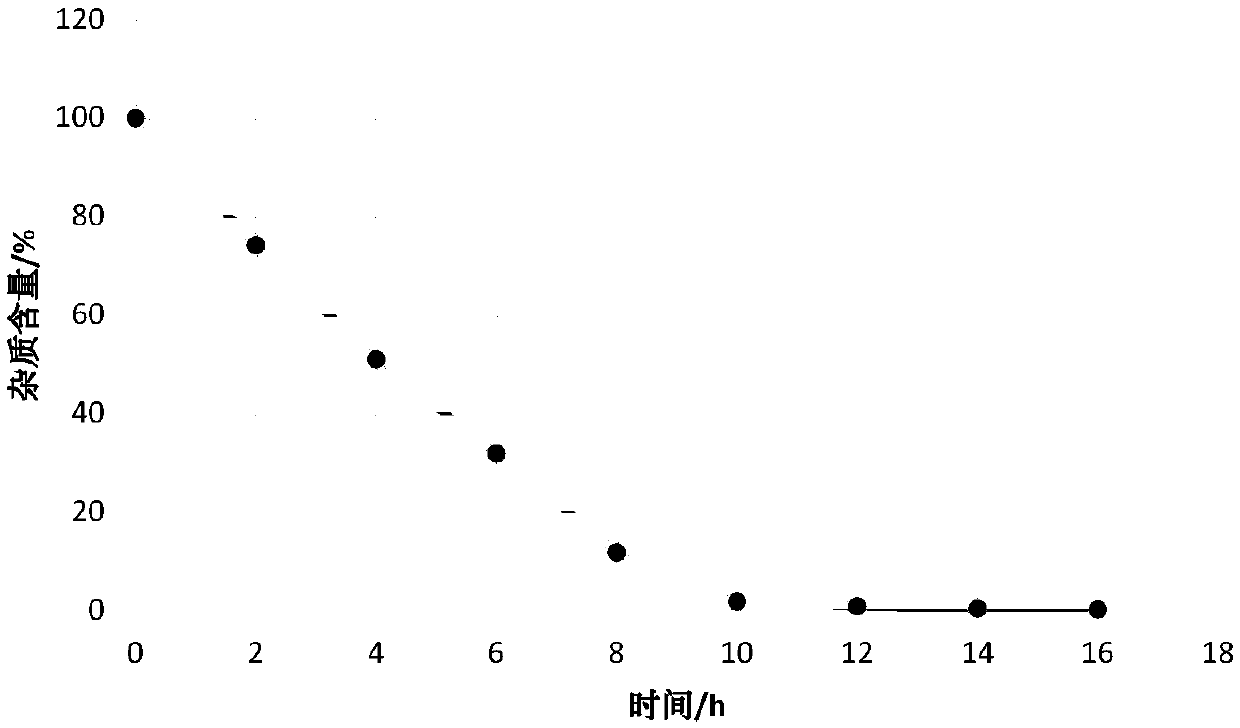
[0025] Example 1: Under the protection of argon, 14.4 g of m-difluorobenzene was added to 20 ml of dichloromethane, and the temperature dropped to 10±5°C, and 14.17 g of trimethylsilyl trifluoromethanesulfonate was added dropwise in batches to control The temperature in the system is 8±3°C, add 6.5g of N-acetylpiperidine-4-formyl chloride into the system, raise the temperature of the system to 35±5°C and keep it for 11 hours, add 30ml of 6mol / L hydrochloric acid solution for washing, and add bicarbonate Wash with 30 ml of sodium solution, add 30 ml of purified water for washing, and concentrate under reduced pressure to obtain 7.1 g of product with a purity of 98.95%.
Embodiment 2
[0026] Example 2: Under the protection of argon, 14.4g of m-difluorobenzene was added to 20ml of dichloromethane, the temperature dropped to 10±5°C, 9.05g of boron trifluoride ether was added dropwise, and the internal temperature of the system was controlled at 8±5°C Add 6.5g of N-acetylpiperidine-4-formyl chloride to the system at 3°C, raise the temperature of the system to 35±5°C and keep it warm for 11 hours, add 30ml of 6mol / L hydrochloric acid solution to wash, add 30ml of sodium bicarbonate solution to wash, Add 30ml of purified water for washing, and concentrate under reduced pressure to obtain 6.7g of product with a purity of 98.42%.
Embodiment 3
[0027] Example 3: Under the protection of argon, 14.4g of m-difluorobenzene was added to 20ml of dichloromethane, the temperature dropped to 10±5°C, 8.5g of aluminum trichloride was added in batches, and the internal temperature of the system was controlled at 8±3°C , add 6.5g of N-acetylpiperidine-4-formyl chloride to the system, increase the temperature of the system to 35±5°C and keep it warm for 11 hours, add 30ml of 6mol / L hydrochloric acid solution for washing, add 30ml of sodium bicarbonate solution for washing, add purification Washed with 30ml of water and concentrated under reduced pressure to obtain 4.7g of product with a purity of 94.95%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com