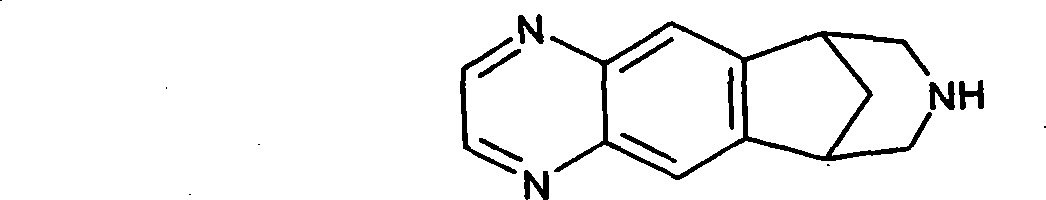
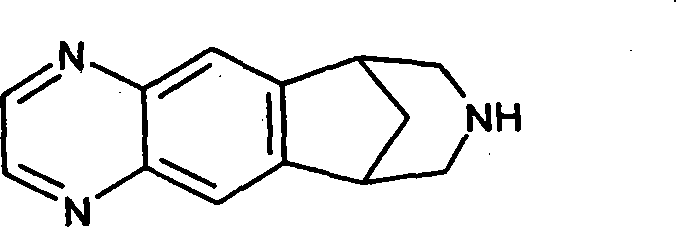
Transdermal system for varenicline
A transdermal, skin-based technology, applied in the field of pharmaceutical compositions for medical use, can solve problems such as reactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] As a method for determining the skin penetration properties of the active ingredient, a human cadaver skin membrane and a receiving liquid such as 1.0 M phosphate buffered saline are used for the Franz diffusion cell. Fill the receiving chamber of the Franz diffusion cell with receiving liquid and maintain the diffusion cell at 34.5°C. Cut off human corpse skin to provide a surface area of 1.767cm 2 的膜。 The film. The HPLC analysis of the liquid is used to determine the amount of active ingredient that penetrates through the membrane at different times ranging from 6 to 48 hours. Repeat each test several times. Table 1 shows the calculated average skin flow rate and average penetration amount per square centimeter for formulations I and II after 24 to 48 hours. These test results obtained by the Franz diffusion cell method show that, based on the theoretical active ingredient, 0.3 to 5 μg / cm 2 The skin flow rate per hour can realize the transdermal delivery of the compound...
Embodiment 2
[0081] Example 2-Matrix type transdermal
[0082] The active ingredient was mixed with an aqueous dispersion of NACOR 72-9965 (National Starch's hydrophobic acrylic copolymer) to achieve a concentration of 2% (w / w) in the dried film after the film was molded. The adhering mixture was shaped onto the released coating polymer film (Rexam Release Technologies; W. Chicago, IL), dried in a convection oven at 60°C, and cut to achieve a 2 mgA dose of active ingredient. The dried film was laminated into a laminate of polyester film (SCOTCHPACK #1012, 3M Pharmaceuticals; St. Paul, MN).
Embodiment 3
[0083] Example 3-Matrix type transdermal system
[0084] (1) Dissolve or disperse the alkali form or salt form of the active ingredient in a polyacrylate solution, such as Duro-Tak(R) 387-2052 adhesive. Add appropriate solvents, accelerators and / or fillers to the adhesive dispersion and mix well. The gas is removed from the resulting mixture and laminated to a release liner such as Medirelease(R) 2228 to form a coating with a thickness of 0.5-2 mm. The adhesive layer is dried at room temperature for 5-10 minutes, and then dried at 40-80°C for 15-30 minutes to remove all volatile solvents. A backing sheet such as Mediflex(R) 1200 is applied to the adhesive side. Store the resulting patch of the desired size in a closed package.
[0085] (2) The base form or the salt form of the active ingredient is dissolved or dispersed in an isobutylene (PIB)-based adhesive such as Duro-Tak(R) 87-6173. The following method is similar to that described in the previous section.
[0086] (3) Dissolv...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com