Preparation methods of varenicline intermediate, varenicline and its salt
A technology of varenicline and acid salt, applied in the field of drug synthesis, can solve the problems of unfavorable target drug product quality control, reduce unit product production cost, increase operation complexity, etc., to ensure product quality, reduce production cost and time cost, shortened response time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
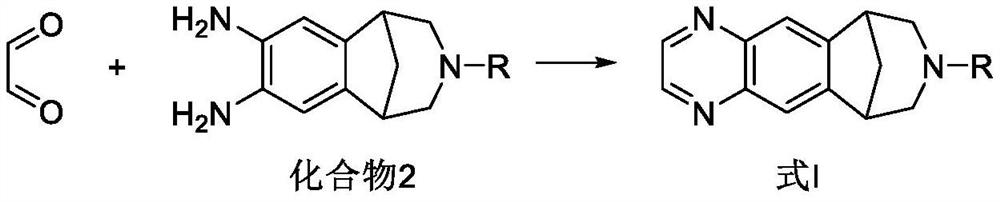
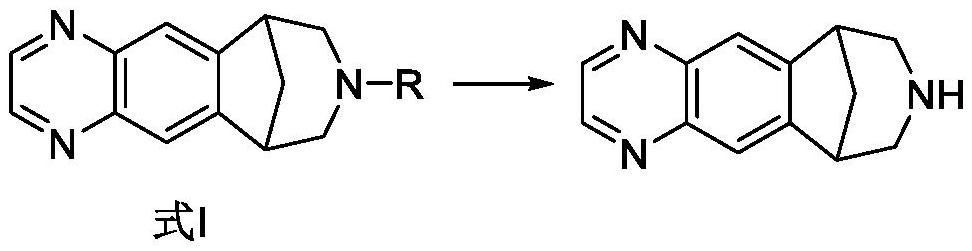
[0140] 1. Preparation of varenicline intermediate
[0141]
[0142] 1. Add 3kg (about 8.69mol) of compound A and 0.35kg of palladium carbon (Pd / C, Pd 5%) catalyst into a reactor containing 36kg of isopropanol and 15kg of purified water, and control the reaction temperature at 25 to 35°C. Pass hydrogen and stir to react for 6h, then stop the reaction (the remaining amount of compound A in the reaction solution detected by HPLC is ≤0.5%), filter the palladium carbon catalyst with a pad of diatomaceous earth, and obtain a reaction solution containing compound B;
[0143]
[0144] 2. Under the protection of an inert gas (the present embodiment is nitrogen), in the reaction solution obtained in step 1., add 75g sodium bicarbonate (about 0.89mol, showing alkalinity), then slowly add the aqueous solution of glyoxal (0.556kg (about 9.58mol) glyoxal and 6.5kg water, showing acidity), control the reaction temperature at 20-30°C, stir the reaction for 8h (including the addition tim...
Embodiment 2
[0157] The same content as in Example 1 is not repeated, but the difference is that in the preparation method of varenicline intermediate, in step 1., the amount of solvent is changed to 30kg (21.2kg isopropanol and 8.8kg purified water), to obtain varenicline The yield of the blue intermediate (compound C) is in the range of 85% to 90% (based on compound A), and the purity detected by HPLC is 99.69%.
Embodiment 3 and 4
[0159] The same content as in Example 1 is not repeated, but the difference is that in the preparation method of varenicline intermediate, in step 1., the consumption of palladium carbon (Pd / C, Pd 5%) catalyst is changed to 0.18kg, 2.8kg respectively. kg to obtain the varenicline intermediate (compound C), the yields were all in the range of 85% to 90% (calculated as compound A), and the purity was 99.19% and 99.03%, respectively, as detected by HPLC.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com