Preparation method for N-nitrosamine genotoxic impurity of varenicline tartrate
A technology of varenicline tartrate and genotoxicity, applied in the direction of organic chemistry and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
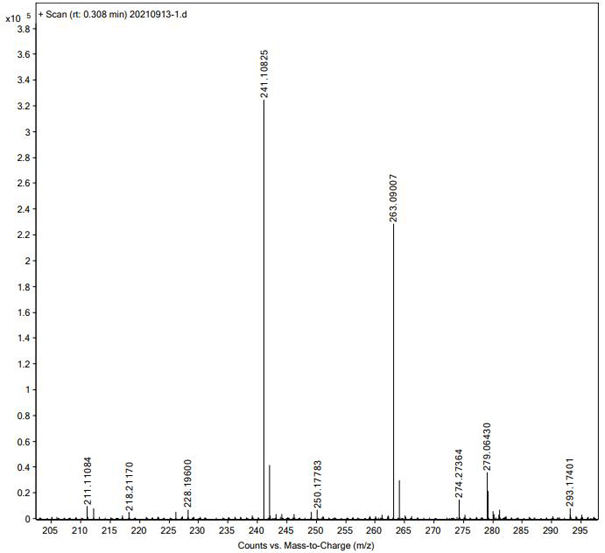
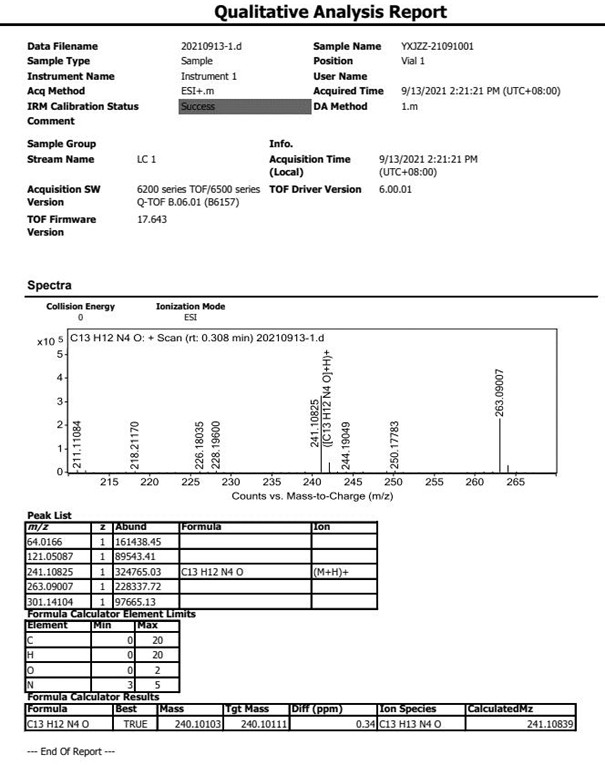
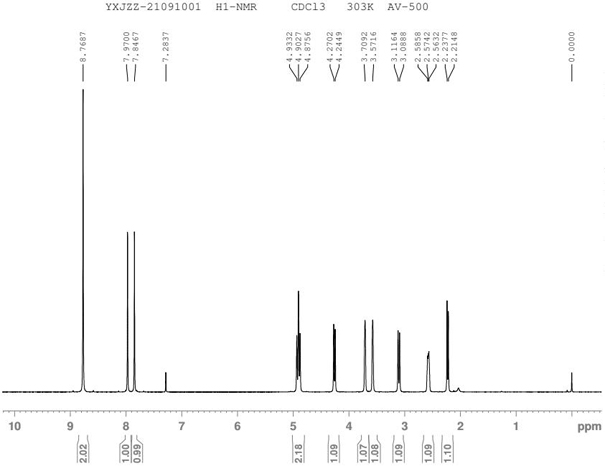
[0020] The technical solution of the present invention will be further described below in conjunction with the accompanying drawings. The embodiments and related experimental data described below with reference to the figures are only exemplary, and are intended to explain the present invention, but should not be construed as limiting the present invention. The disclosure below provides many different embodiments to be used for realizing technical effect of the present invention, in order to simplify the disclosure of the present invention, only provide several kinds of embodiments that adopt specific experimental parameters hereinafter, but those of ordinary skill in the art can realize Application of other processes and / or use of other materials. In this specification, references to a schematic representation of an embodiment do not necessarily refer to the same implementation or example. Furthermore, specific features or features described may be combined in any suitable m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com