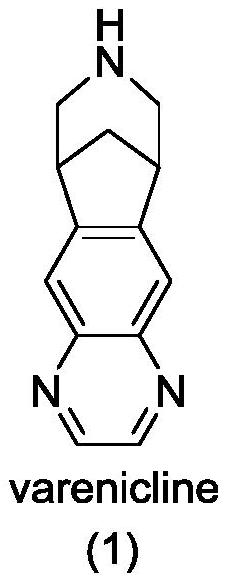
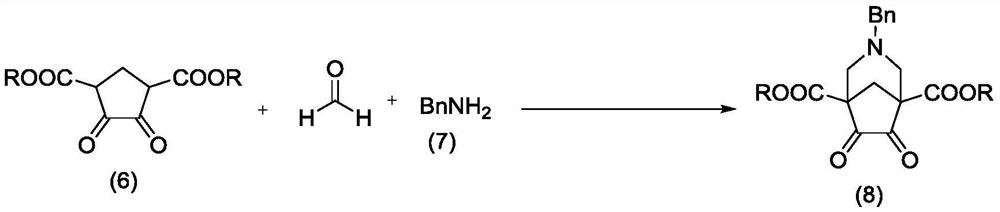
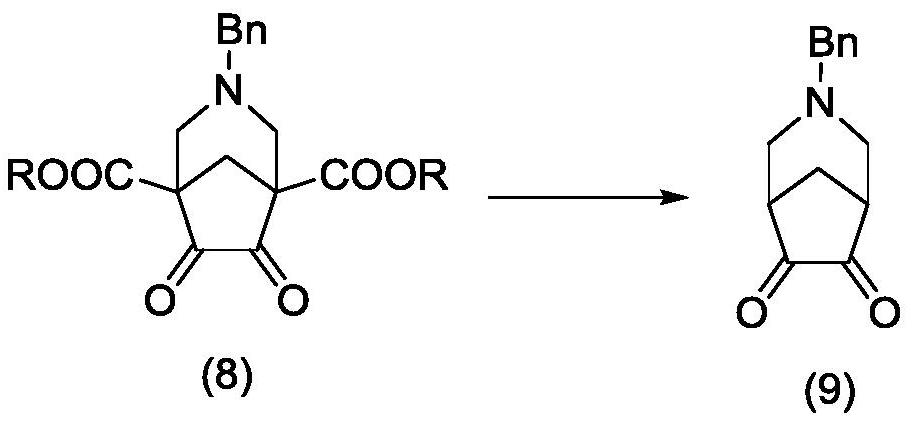
Varenicline synthesis method
A technology for varenicline and compounds, applied in the field of preparation of intermediate compounds, can solve problems such as inconvenience, safety problems, operating conditions, expensive reagents and/or catalysts, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0045] The following examples provide detailed experimental procedures and parameters suitable for the preparation of varenicline or a pharmaceutically acceptable salt thereof according to the present invention and are intended to be illustrative and not limiting.
[0046] Unless otherwise stated, all materials, solvents and reagents, including anhydrous solvents such as DMF and DCM, were obtained from commercial suppliers in the best grade and used without further purification. All reactions involving air- or moisture-sensitive compounds were performed under a nitrogen or argon atmosphere unless otherwise stated.
[0047] 1 H(400MHz) and 13 C NMR (100MHz) data using CDCl 3 or DMSO-D 6 Obtained as a solvent on Bruker AVANCE II 400MHz. Chemical shifts (δ) are in ppm and coupling constants (J) are in Hz. 1 H NMR spectrum is recorded with tetramethylsilane (δ=0.00ppm) as an internal reference; 13 C NMR spectrum with CDCl 3 (δ=77.00ppm) or DMSO-D 6 (δ = 39.5 ppm) was recor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com