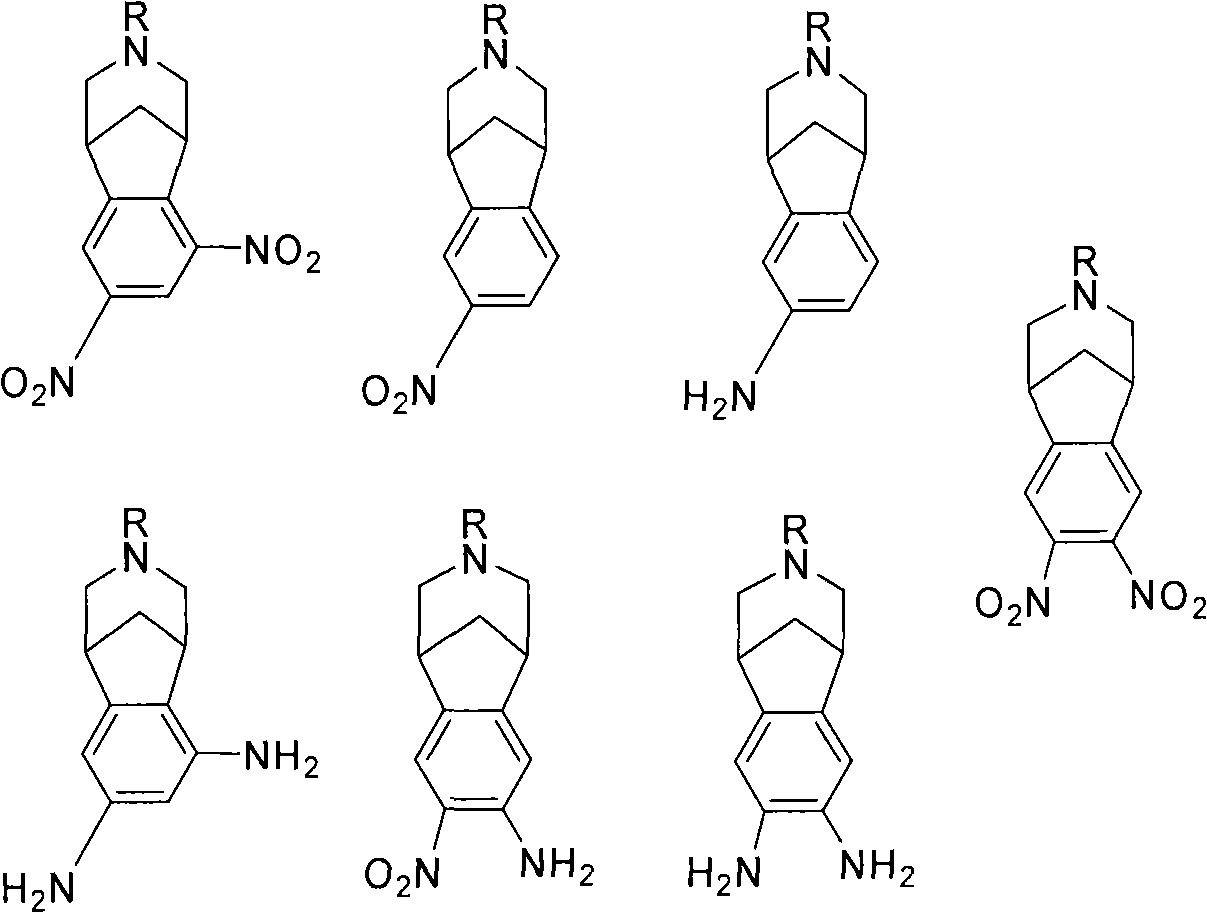
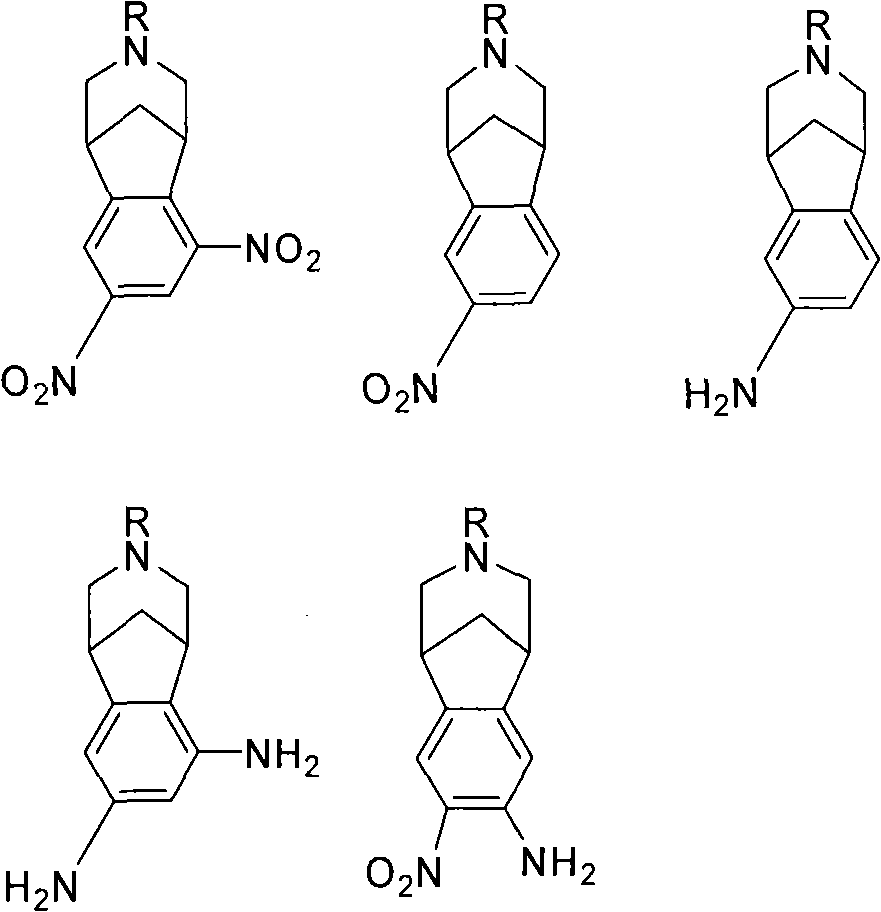
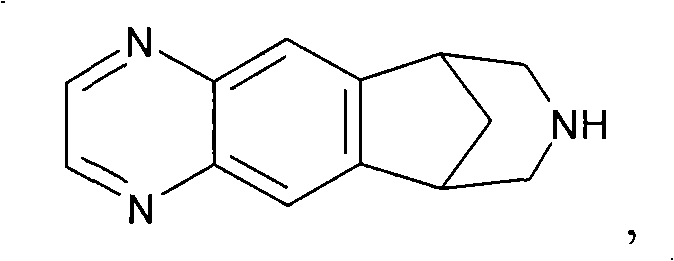
Varenicline standards and impurity controls
A composition and drug technology, applied in the fields of cognitive dysfunction, amyotrophic lateral sclerosis, jet lag, and sleep disorders, can solve cumbersome problems and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0088] Part A: Example Clearance Experiments
[0089] Experimental Determination of Fold Clearance: Synthesis of Varenicline
[0090] Compound 8 was slurried in toluene and added to an aqueous solution of NaOH (3.1 equiv). The biphasic mixture was heated to 37-40°C, whereupon two clear yellowish layers resulted. At this point, 50 ppm of compound 3 (R=H) was spiked into the reaction mixture. The conversion of compound 8 to varenicline (9) was then carried out, including treating the biphasic toluene / water phase with Darco KB-B and recovering the compound 9 / toluene solution. The compound 9 / toluene solution was azeotroped with MeOH. The resulting compound 9 / methanol solution was added to the L-(+)-tartaric acid / MeOH solution to form salt 10. After the granules were formed, Salt 10 was filtered and dried.
[0091] The isolated material was analyzed for residual compound 3 (R=H). When a temperature of 37-40°C was reached, 50 ppm of Blend 3 was introduced into the reaction mix...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com