Varenicline tartrate intermediate impurity and preparation method thereof
A technology for varenicline tartrate and its intermediates, which is applied in organic chemistry and other fields, can solve the problem of no impurity I preparation method, etc., and achieve the effect of improving the identification and separation of impurities and improving product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
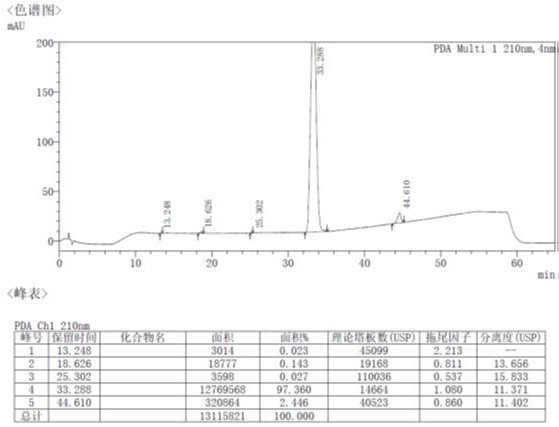
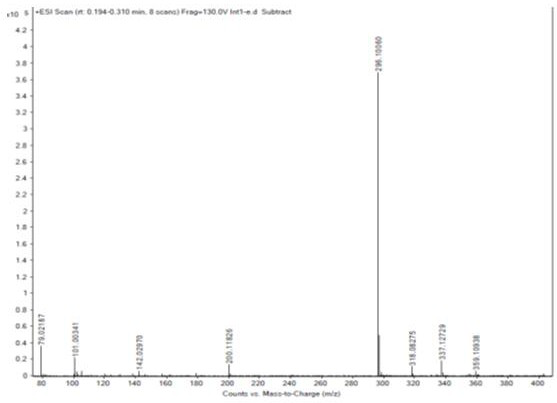
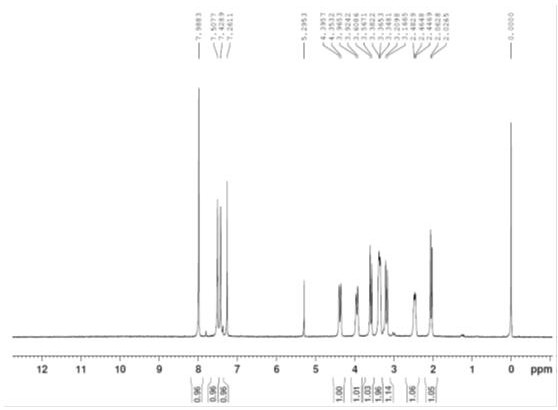
[0025] The technical solutions of the present invention are further described below with reference to the accompanying drawings. The embodiments and related experimental data described below with reference to the accompanying drawings are only exemplary, and are intended to be used to explain the present invention, but should not be construed as a limitation of the present invention.
[0026] 1. Preparation of Intermediate 1
[0027] Add 250ml of dichloromethane and 25g of starting materials to the reaction flask, turn on stirring, cool down to 0~5°C, add 31.0g of triethylamine in the system, and add 32.2g of trifluoroacetic anhydride dropwise to the system at 0~5°C After dropping, the temperature was raised to 20-30°C, and the reaction was stirred for 2 h. 250 ml of water was added to the reaction solution, stirred for 1 h, allowed to stand, layered, and the organic phase was collected. The aqueous phase was extracted with 250 ml of dichloromethane, and the organic phases wer...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com