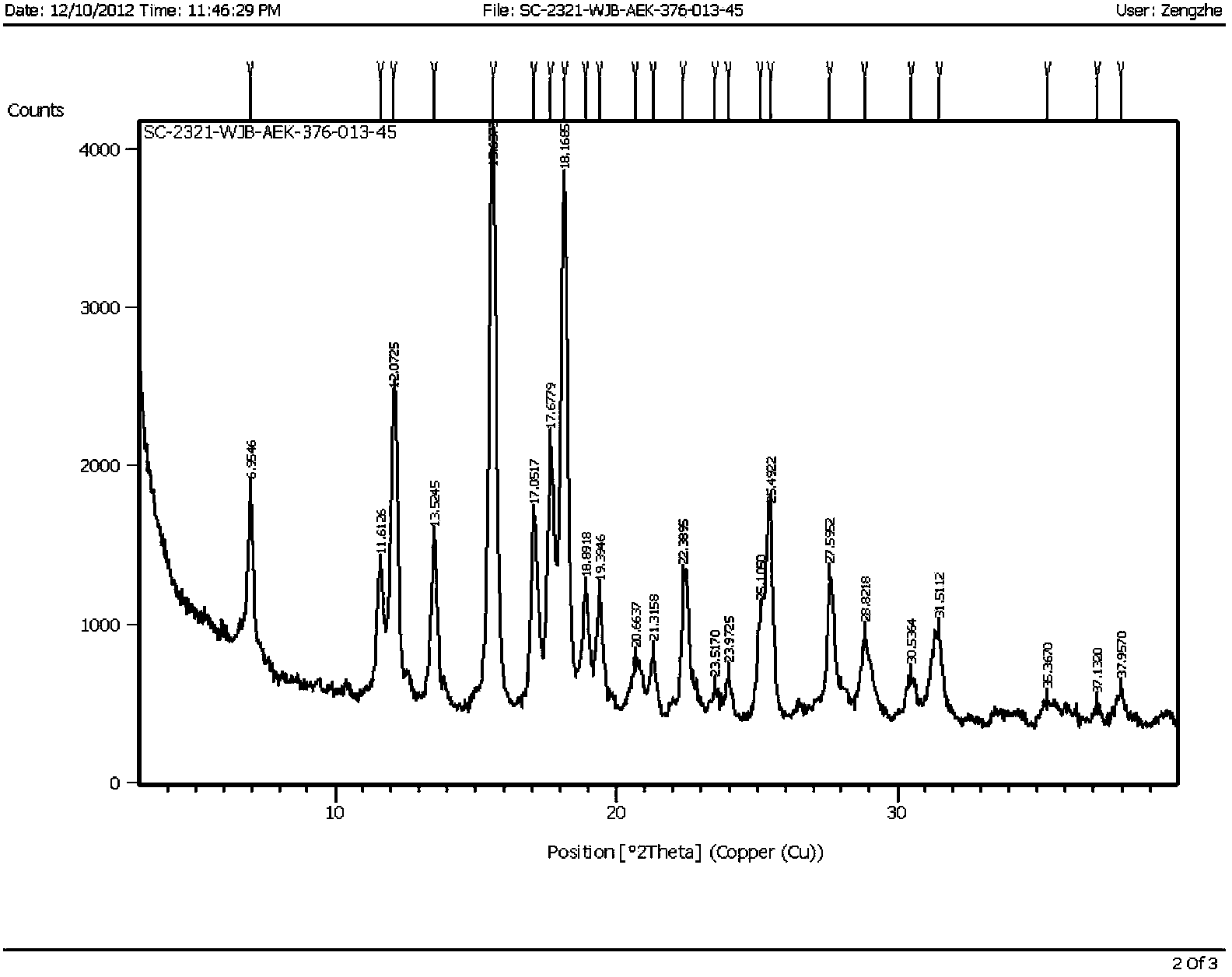
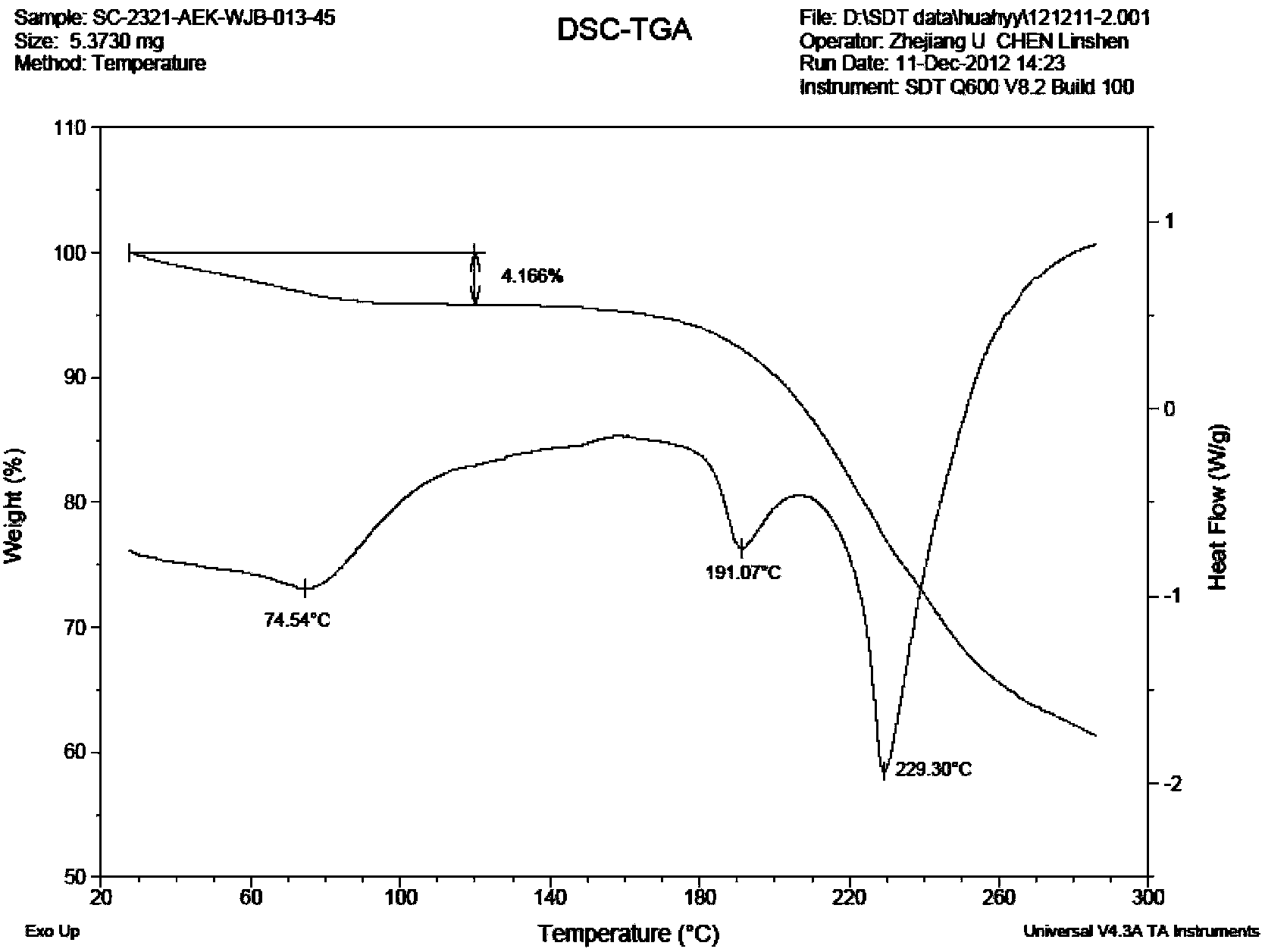
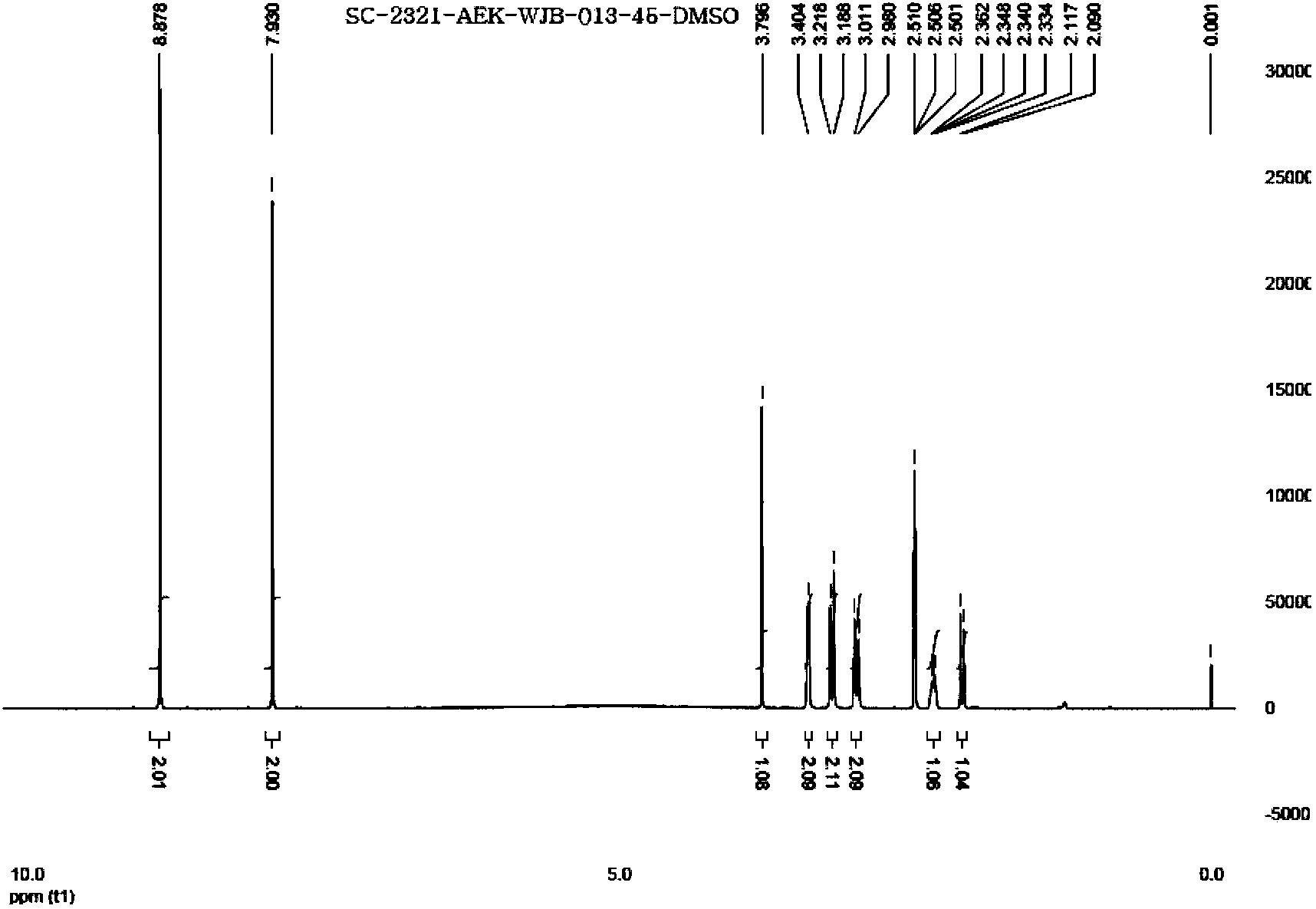
Varenicline salt and preparing method thereof
A varenicline and tartrate technology, applied in the field of anti-nicotine addiction drug varenicline salt and its preparation, can solve the problems of performance such as solubility and thermal stability without detailed evaluation and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Dissolve 3g of varenicline in 25ml of ethanol, dissolve 1.02g of tartaric acid in 10ml of ethanol, slowly add the ethanol solution of L-tartaric acid into the ethanol solution of varenicline under stirring, after the addition is complete, heat up to 70°C, Add 4.5ml of water until it is just clear, maintain this temperature for 10 minutes, let it stand still, a large amount of off-white solid precipitates, filter it with suction, and dry it in vacuum to obtain 2.8g of off-white solid, with a yield of 68.9%.
Embodiment 2
[0040] Dissolve 3g of varenicline in 20ml of acetone, dissolve 1.02g of tartaric acid in 5ml of water, slowly add the aqueous solution of L-tartaric acid into the acetone solution of varenicline under stirring, after the addition is complete, heat up to 55°C, add 2.6 ml of water until it is just clear, maintain this temperature for 10 minutes, let it stand still, a large amount of off-white solid precipitates, filter it with suction, and dry it in vacuum to obtain 3.3 g of off-white solid, with a yield of 81.2%.
Embodiment 3
[0042] Dissolve 3g of varenicline in 20ml of methanol, dissolve 1.02g of tartaric acid in 5ml of methanol, slowly add the methanol solution of L-tartaric acid into the methanol solution of varenicline under stirring, after the addition is complete, heat up to 65°C, Add 1.5ml of water until it is just clear, maintain this temperature for 10 minutes, let it stand still, a large amount of off-white solid precipitates, filter it with suction, and dry it in vacuum to obtain 2.5g of off-white solid, with a yield of 61.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com