Method for detecting nitrosamine genotoxic impurities in varenicline intermediate
A detection method and a genotoxicity technology are applied in the field of detection of nitrosamine genotoxic impurities in varenicline intermediates, which can solve the problems of scarcity of detection methods, safety of varenicline medication, etc., and reduce the side effects of medication in patients. Response, avoidance of clinical risk, and the effect of improving detection sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Embodiment 1: method groping
[0061] (1) Chromatographic conditions
[0062] The filler is octadecyl bonded silica gel column,
[0063] The mobile phase is 0.1% formic acid aqueous solution and 0.1% formic acid acetonitrile solution with different volume ratios, and the gradient elution is carried out according to the following table
[0064]
[0065]
[0066] The column temperature is 40°C,
[0067] The mobile phase flow rate is 0.4mL / min,
[0068] The detection wavelength is 214nm.
[0069] (2) Mass Spectrometry Conditions
[0070] The ion source is a heatable electrospray ionization source, and the ion source temperature is 450°C
[0071] The sheath gas flow rate is 55 arbitrary units, and the auxiliary gas flow rate is 15 arbitrary units.
[0072] Collision energy is 50~70(NCE)
[0073] The temperature of the ion transfer tube was 380°C.
[0074] The scan mode is positive ion mode,
[0075] The acquisition mode is parallel reaction monitoring mode. ...
Embodiment 2
[0084] Example 2 Methodological investigation - system applicability
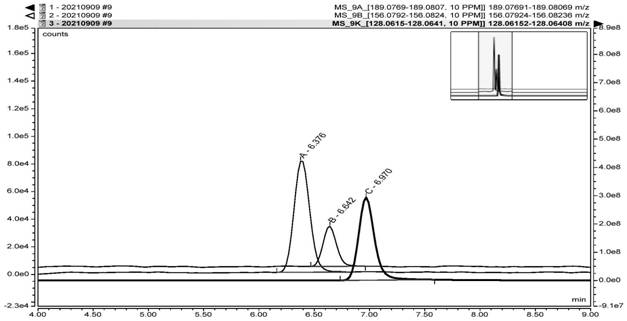
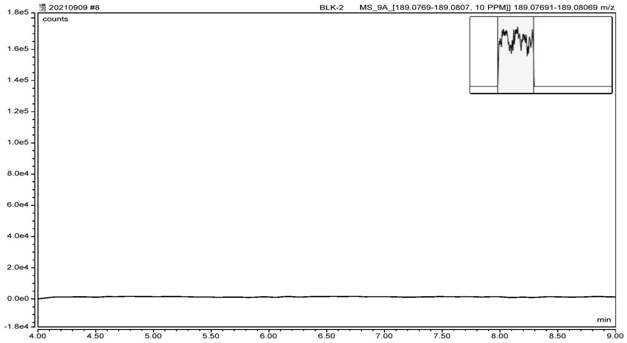
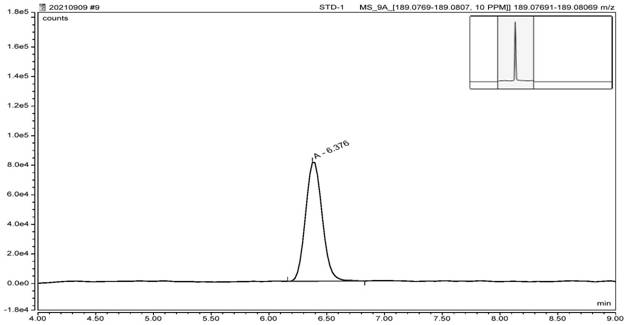
[0085] According to the expert model and the reasoning of the software, it is predicted that the genotoxic impurities A, B, and C are all three types of impurities in ICH. According to the guidelines of ICH M7, it is necessary to carry out impurity research and control. Based on the toxicological threshold (TTC) data of 1.5 μg / day and actual process conditions, it was finally confirmed that the limits of genotoxic impurities A, B, and C were all 3.9 ppm. According to this limit and the solubility of varenicline intermediates, 1 mL of reference solution containing 7.8 ng of genotoxic impurities A, B, and C were prepared respectively. After the system was balanced, 6 injections were repeated, and the mass spectrum was recorded. The system is applicable. The test results are shown in Table 1.
Embodiment 3
[0086] Embodiment 3 methodological investigation-limit of quantitation
[0087] According to the prepared reference substance solution in Example 1, it was diluted 5 times, and it was prepared into a quantitation limit solution containing 7.8ng genotoxic impurity A, 7.8ng genotoxic impurity B and 7.8ng genotoxic impurity C, and the signal-to-noise ratio of each impurity was equal to Greater than 10, the quantitative limit test results are shown in Table 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Collision energy | aaaaa | aaaaa |
| Linear correlation coefficient | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com