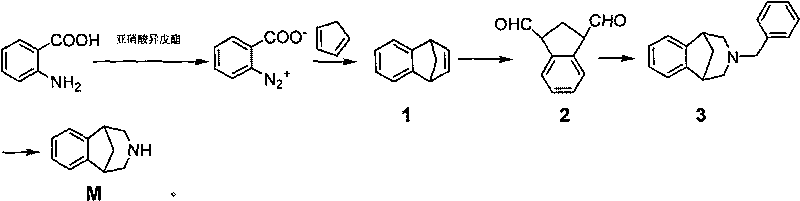
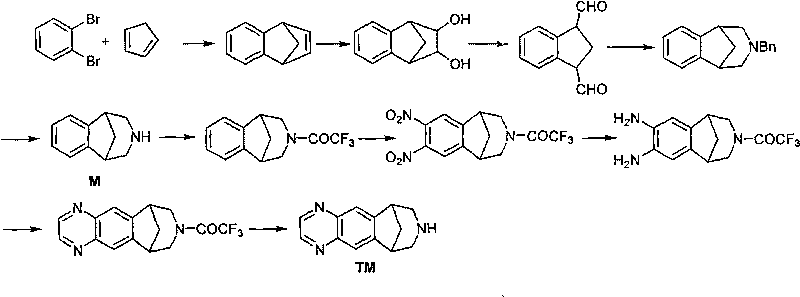
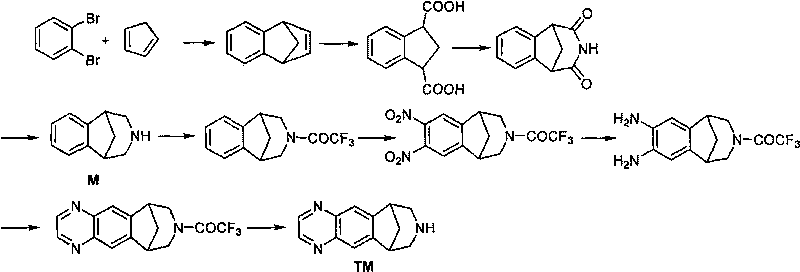
Method for synthesizing Varenicline intermediate 2, 3, 4, 5-tetralin-1, 5-methylene-hydrogen-benzoazepine
An intermediate, methylene technology, applied in the field of synthesis of organic intermediates, can solve the problems of difficult separation, environmental pollution, high cost, etc., and achieve the effects of simple production process, simplified method, and reduced cost of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] A method for synthesizing varenicline M intermediate 2,3,4,5-tetrahydro-1,5-methylene-hydrogen-benzazepine, comprising the steps of:
[0040] The first step: take a 2L four-necked bottle (mechanical stirring, thermometer, constant pressure dropping funnel), add 137g (1 mole) anthranilic acid, 2.4g (0.015 mole) trichloroacetic acid, 1L tetrahydrofuran (THF) solvent, Place it in an ice-salt bath, add 192g (1.64 moles) of isoamyl nitrite dropwise under stirring, and drop it in about 10 minutes. After dropping, remove the ice-water bath, place the reaction bottle at room temperature, and continue to stir for 2 h. Cool the reaction solution to about 0°C with an ice-water bath, filter under reduced pressure to obtain the diazonium salt filter residue, wash it with cold THF until the wash solution becomes colorless, and then wash it with 1L of cold CH 2 Cl 2rinse. Carefully transfer the wet diazonium salt slurry to a 1L four-neck flask (with mechanical stirring, reflux cond...
Embodiment 2
[0047] The first step: take a 2L four-necked bottle (mechanical stirring, thermometer, constant pressure dropping funnel), add 137g anthranilic acid, 2.4g trichloroacetic acid, 1L Me-THF solvent, place in an ice-salt bath, stir 192g of isoamyl nitrite was added dropwise, and the drop was completed in about 10 minutes. After dropping, remove the ice-water bath, place the reaction bottle at room temperature, and continue stirring for 2 hours at a temperature of 18-20°C. The reaction solution was cooled to about 0°C with an ice-water bath. Filtrate under reduced pressure, wash the filter residue with cold Me-THF until the wash solution becomes colorless, then wash with 1L cold CH 2 Cl 2 rinse. Carefully transfer the wet slurry to a 1L four-neck flask (with mechanical stirring, reflux condenser, thermometer), add 2.5L CH 2 Cl 2 , 99g of newly prepared cyclopentadiene, stirred at 39.5°C for about 1.5h, stopped the reaction, concentrated to remove the solvent, and then changed ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com