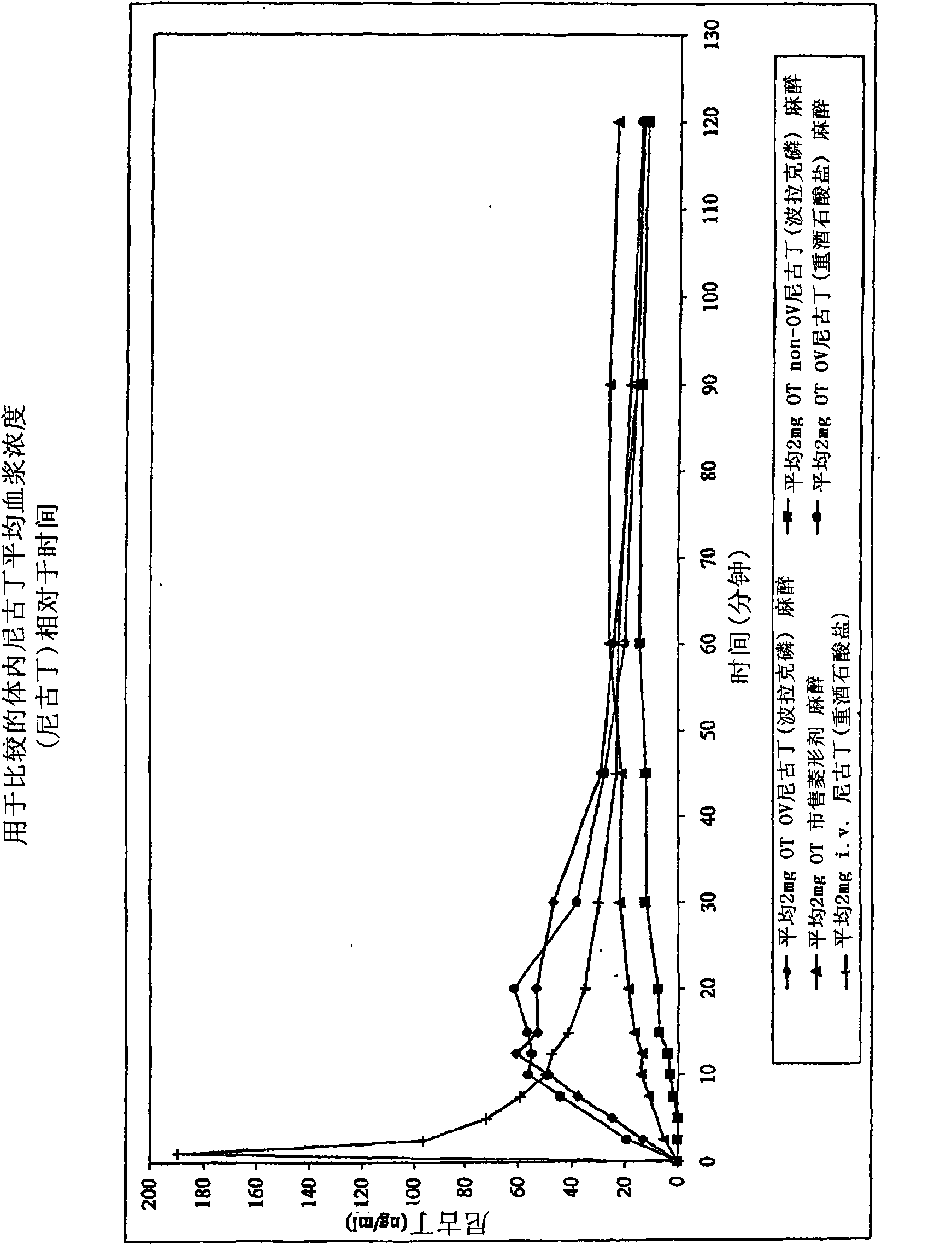
Oral transmucosal nicotine dosage form
A technology of nicotine and dosage forms, which is applied in the fields of pill delivery, nervous system diseases, and oral medicine feeding equipment, etc. It can solve the problems of delay in the systemic acceptance of active ingredients and the "thinning" of active ingredients, and achieve the effect of convenient tablet size
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Example 1: Preparation of a packaged oromucosal dosage form (tablet) comprising 2 mg nicotine from nicotine polacrol
[0062] A 200 mg solid oromucosal tablet was prepared with a nicotine poracphos potency (15%) effective to deliver a 2 mg dose of active nicotine. Nicotine pollac, mannitol (spray-dried grade), sodium bicarbonate, citric acid, sodium carbonate, and sodium starch glycolate were sieved and blended in a mixer for a predetermined period of time (approximately 30 minutes). After preparing the mixture, magnesium stearate was then added to the mixture and blended for about 5 minutes. The resulting mixture is then discharged and compressed on a rotary tablet press to form tablets to a defined and predetermined weight (200 mg) and hardness (10N). The tablets are then sorted and packed in aluminum-blister packs. Blending, tableting, and blister packaging operations were all performed under humidity-controlled environmental conditions (less than 25 grains of mois...
Embodiment 2
[0067] Example 2: Preparation of a packaged oromucosal dosage form (tablet) comprising 2 mg of nicotine derived from nicotine bitartrate
[0068] Using a procedure similar to that described in Example 1 above, a 200 mg solid oromucosal tablet containing nicotine bitartrate as the active nicotine source was prepared. The composition of the preparation is shown in the table below:
[0069] Table 2: 2 mg Nicotine (from Nicotine Bitartrate) Tablets
[0070] Element:
[0071] * Nicotine bitartrate dihydrate based on 34% potency and 2 mg dose of nicotine.
Embodiment 3
[0072] Example 3: 2 mg nicotine (from nicotine pollac) formulation for comparison
[0073] Using a procedure similar to that described above in Example 1, a 200 mg nicotine tablet formulation was prepared comprising the remaining excipient components preferred for use in the present invention but excluding the effervescent couple and pH adjusting substances of the present invention . The bulking ingredient mannitol was used in place of the effervescent couple and pH adjusting ingredient amounts in the Nicotine Pollack formulation of Example 1. The resulting formulations are shown in the table below:
[0074] Table 3: 2 mg nicotine (from nicotine pollac) formulations for comparison
[0075] Element:
[0076] * Nicotine Pollack Phosphorus is based on 15% potency and nicotine at a dose of 2 mg.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com