Nicotine replacement therapy products comprising synthetic nicotine
A technology for nicotine, products, used in the field of nicotine replacement therapy products containing synthetic nicotine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0098] The following examples are provided for illustrative purposes only and are not intended to limit the scope of any embodiments of the invention.
Synthetic example 1
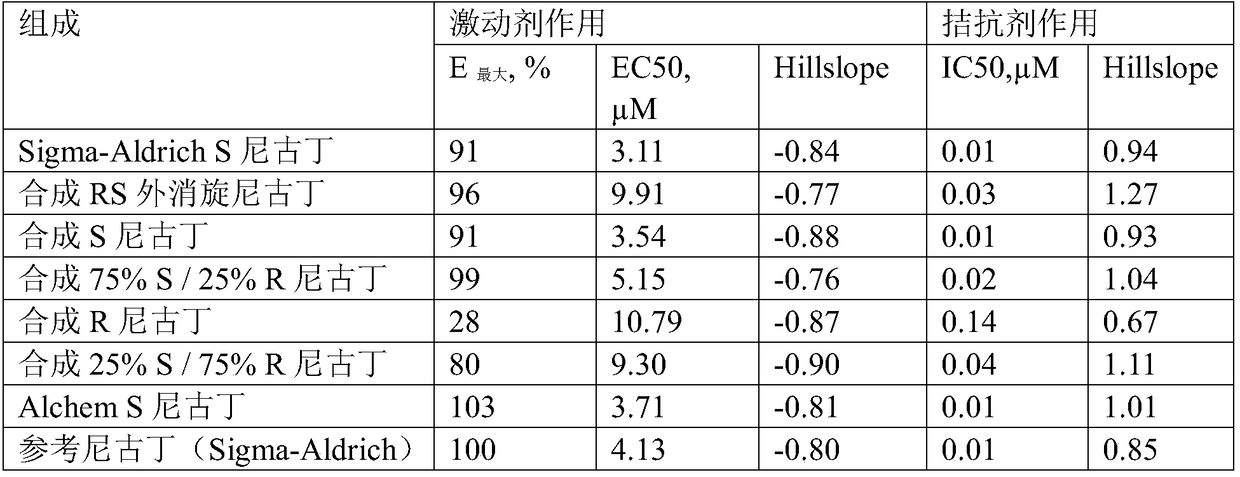
[0099] Synthesis example 1-R, the synthesis of S nicotine
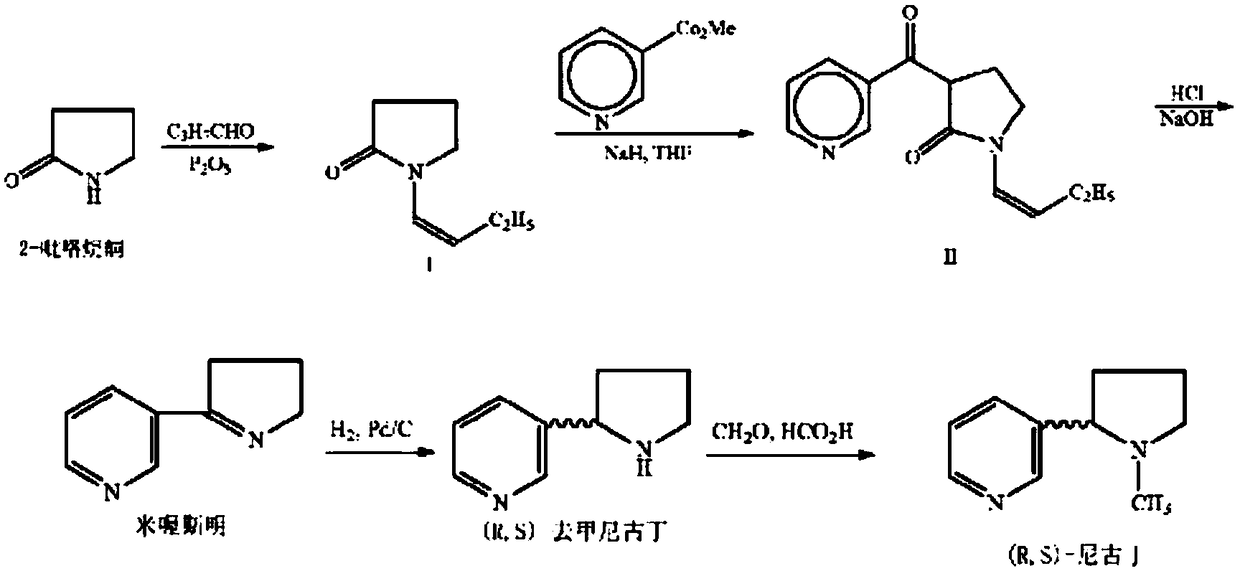
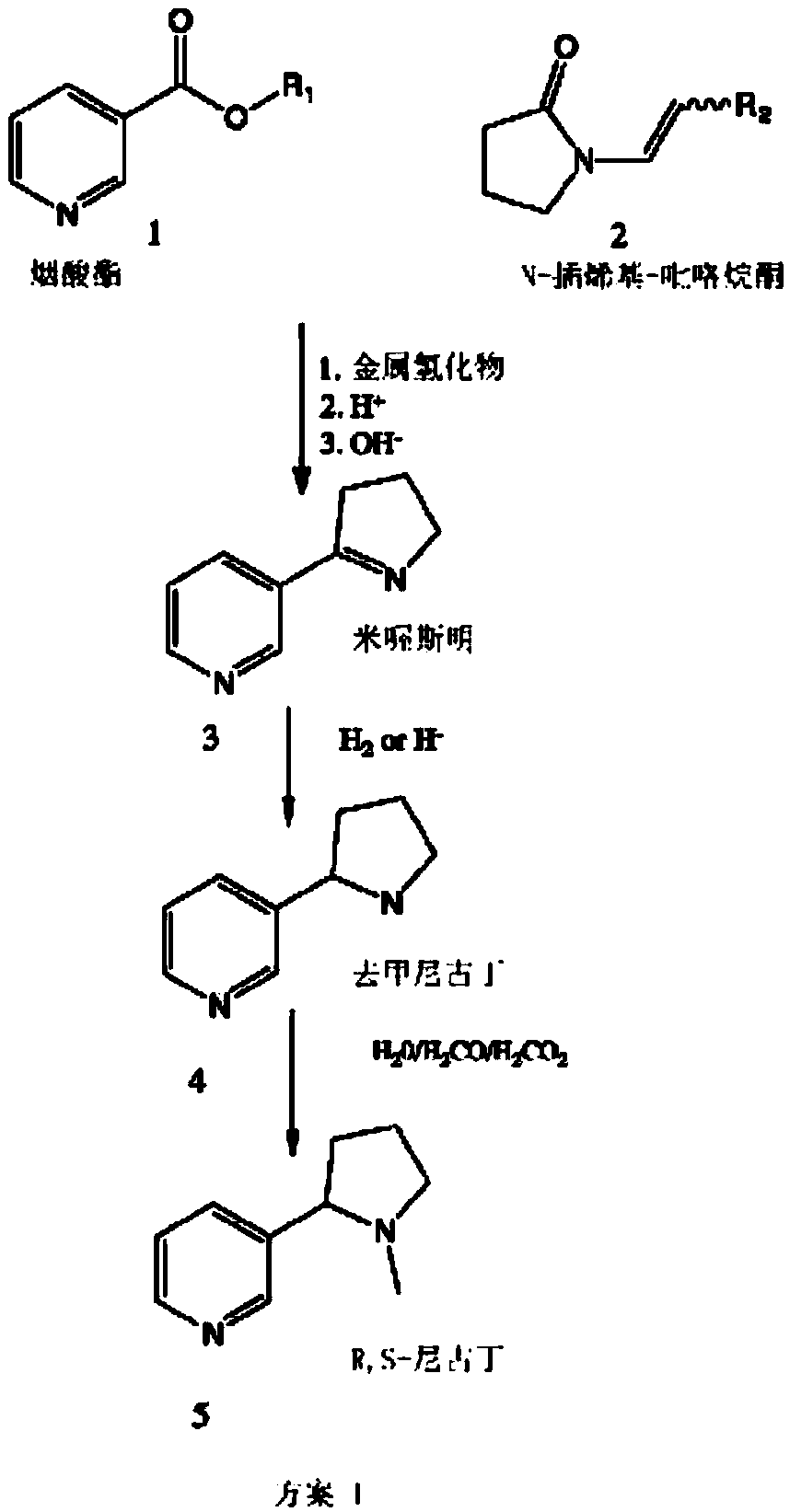
[0100]To a stirred solution of 1-vinyl-2-pyrrolidone (2) in dry THF was added 1 equivalent of potassium hydride under nitrogen atmosphere. The reaction mixture was stirred at room temperature for about 20 minutes, then ethyl nicotinate (1 equiv) was added and the resulting mixture was stirred at 65 degrees Celsius for 24 hours. The reaction was cooled, then acidified using 5% HCl, then concentrated hydrochloric acid was added, and the resulting solution was refluxed for 48 hours. The pH was adjusted to 13 using sodium hydroxide, and the aqueous and organic layers of the resulting biphasic solution were separated 3 times using the same volume of dichloromethane. The combined separated extracts were dried over sodium sulfate, filtered, and the solvent was evaporated to give an amorphous material. The amorphous material was dissolved in 3 parts of ethanol, then palladium on carbon (about 10%) was added, and the resul...
Synthetic example 2
[0101] Synthetic example 2-R, the synthesis of S nicotine
[0102] To a stirred solution of 1-vinyl-2-pyrrolidone (2) in dry THF / DMF (3 / 1 ) was added 1.2 equivalents of sodium hydride under nitrogen atmosphere. The reaction mixture was stirred at room temperature for about 20 minutes, then ethyl nicotinate (1 equiv) was added and the resulting mixture was stirred at 65 degrees Celsius for 24 hours. The reaction was cooled, then acidified using 5% HCl, then concentrated hydrochloric acid was added, and the resulting mixture was refluxed for 48 hours. The pH was adjusted to 6 with sodium hydroxide, then excess dichloromethane was added and the layers were separated. The aqueous layer was extracted twice with excess dichloromethane, and the extracts were combined, washed with water, and dried over sodium sulfate. The solution was then filtered and the solvent was removed using vacuum to give a brown solid. This solid was dissolved in ethanol (about 5 to about 10 parts), then...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com