Therapeutic composition and configuration
A technology for therapeutic compositions and substrates, which can be used in drug delivery, food science, pharmaceutical formulations, etc., and can solve problems such as uneven distribution of subtypes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
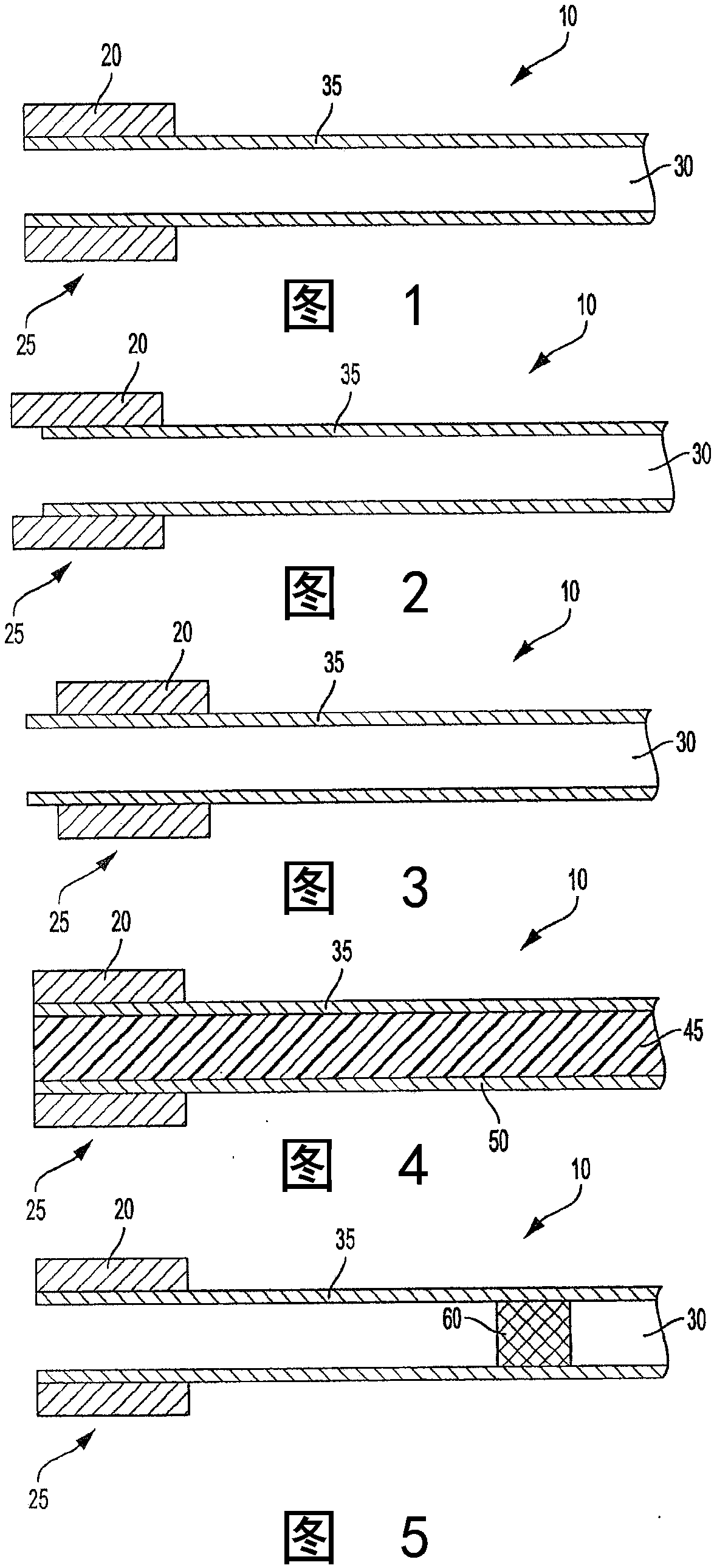
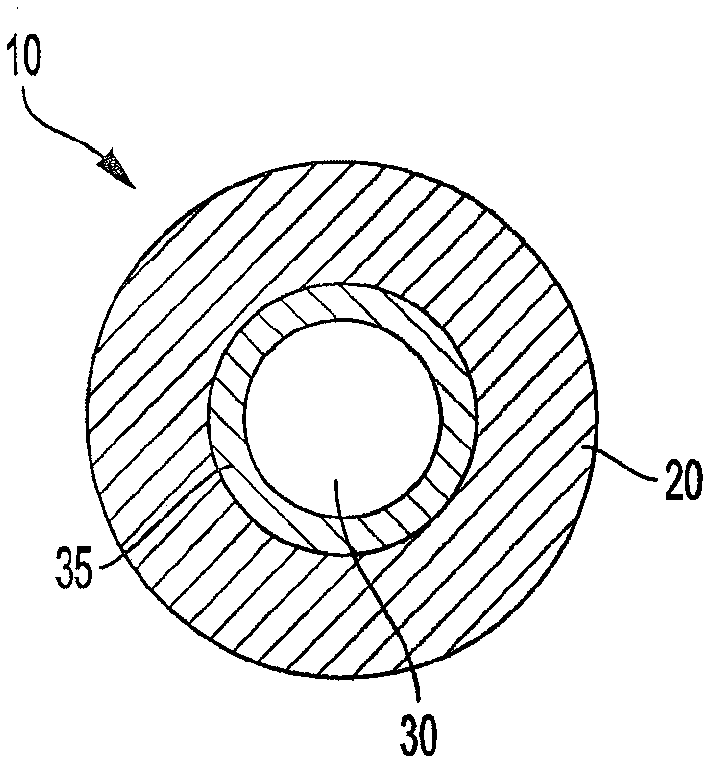
[0097] A lozenge formulation of Nicotine Polacrilex Lozenge 2 mg (nicotine) sold by the company Perrigo is provided. However, rather than providing a generally round and slightly cylindrical tablet with a diameter of about 17 mm, a similar manufacturing technique was used to provide a lozenge having an outermost diameter of about 21 mm and a thickness of about 4 mm. A longitudinally extending passageway with a diameter of about 9 mm is formed or shaped through the center of the lozenge. A tubular substrate of the type sold under the trade name "Favor" is inserted in the passageway of the lozenge. The substrate has a porous plug near its most upstream end, and the plug serves as a carrier for nicotine. The exact size of the central passage of the tablet is such that the tablet and substrate are held securely in place by a friction fit. The lozenge is located in the mouth end region of the substrate such that the most distal mouth end of the lozenge is positioned in alignment ...
Embodiment 2
[0099] A system of the type generally described with reference to Example 1 is provided. However, prior to assembly, the inner surface of the passageway through the lozenge was wetted with distilled water; and the outer surface of the mouth end region of the substrate was wetted with distilled water. The system was then configured by inserting the mouth end of the substrate through the passageway of the lozenge such that the most distal mouth end region of the lozenge was about 4 mm upstream of the most distal mouth end region of the substrate. The resulting system was then dried by subjecting the system to a stream of drying air. The use of moisture and drying of the system serves to provide adhesion or bonding of the tablet to the substrate. Thereby, a system with good physical integrity is provided.
Embodiment 3
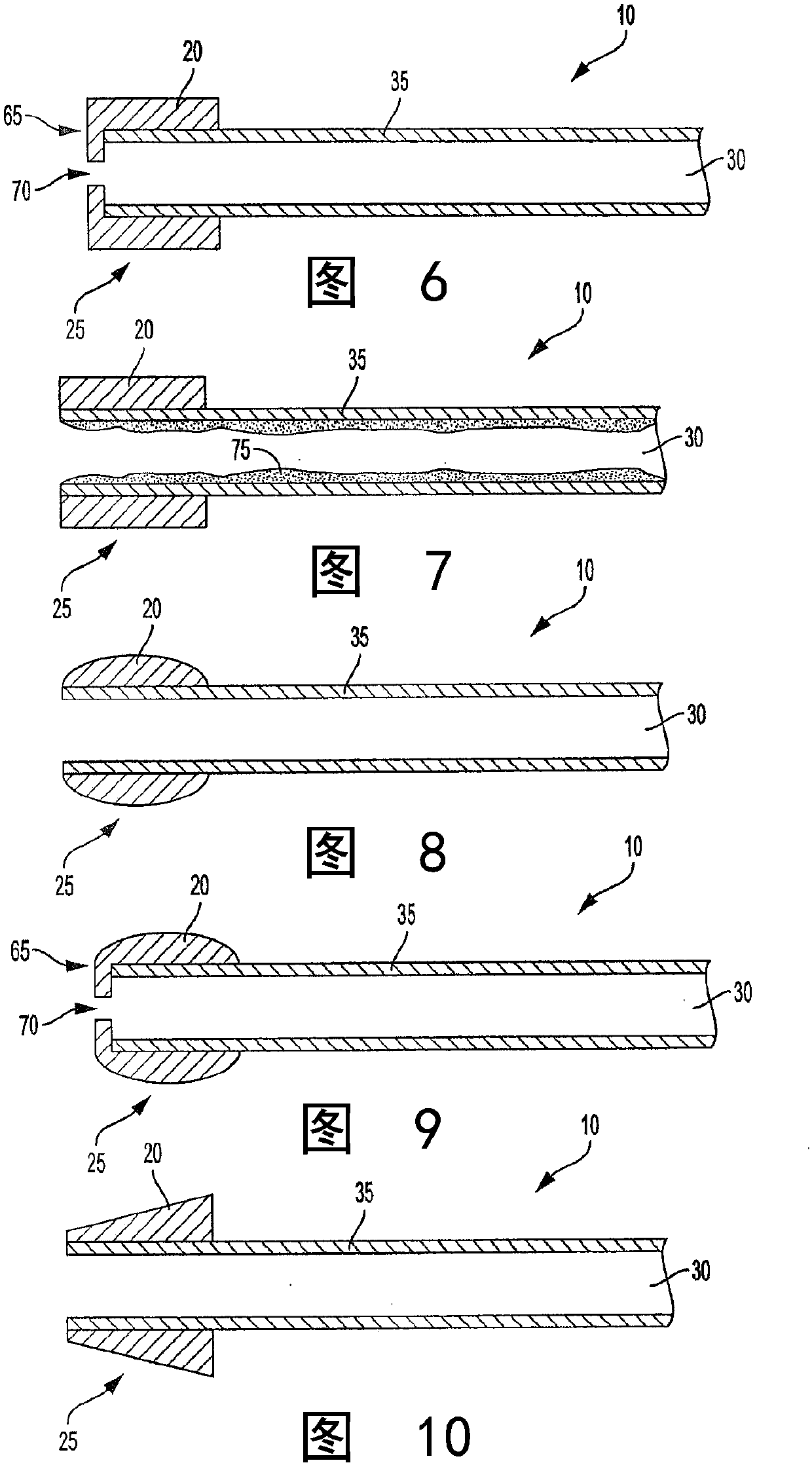
[0101] A lozenge was provided substantially of the type described with reference to Example 1, except that the longitudinally extending passage through the lozenge had a diameter of about 6 mm and the overall diameter of the lozenge was about 20 mm. In addition, the substrate chosen was a tube of about 80 mm in length and about 6.2 mm in diameter. Plastic tubes are provided by cutting plastic straws to the desired length. Straws for providing substrates have been marketed as Home 360° Flexible Straws by DZA Brands, LLC. The system was then configured by inserting one end of the substrate through the passageway of the lozenge such that the most distal mouth end region of the lozenge was about 4 mm upstream of the most distal mouth end region of the substrate. Thus, a system is provided that has a reference image 3 Describes many properties of the system.
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com