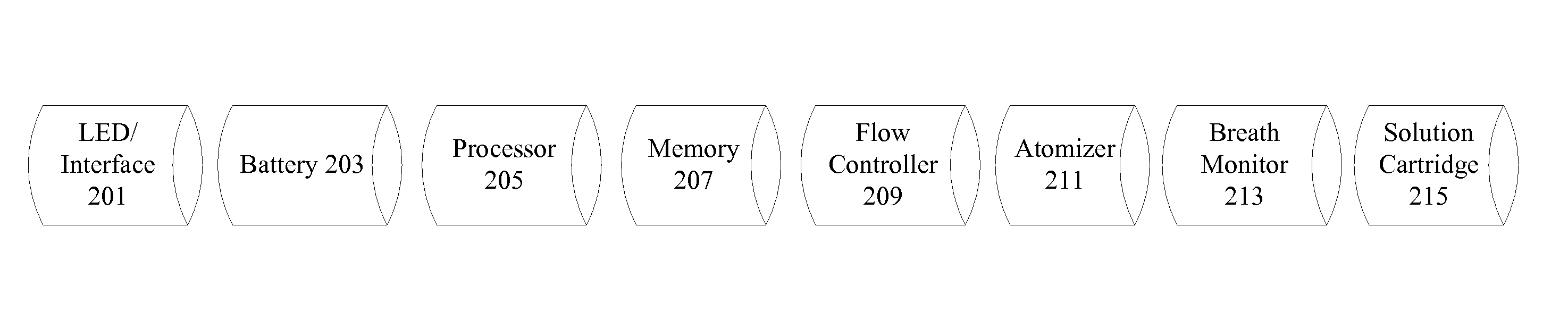Patents
Literature
59 results about "Nicotine delivery" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
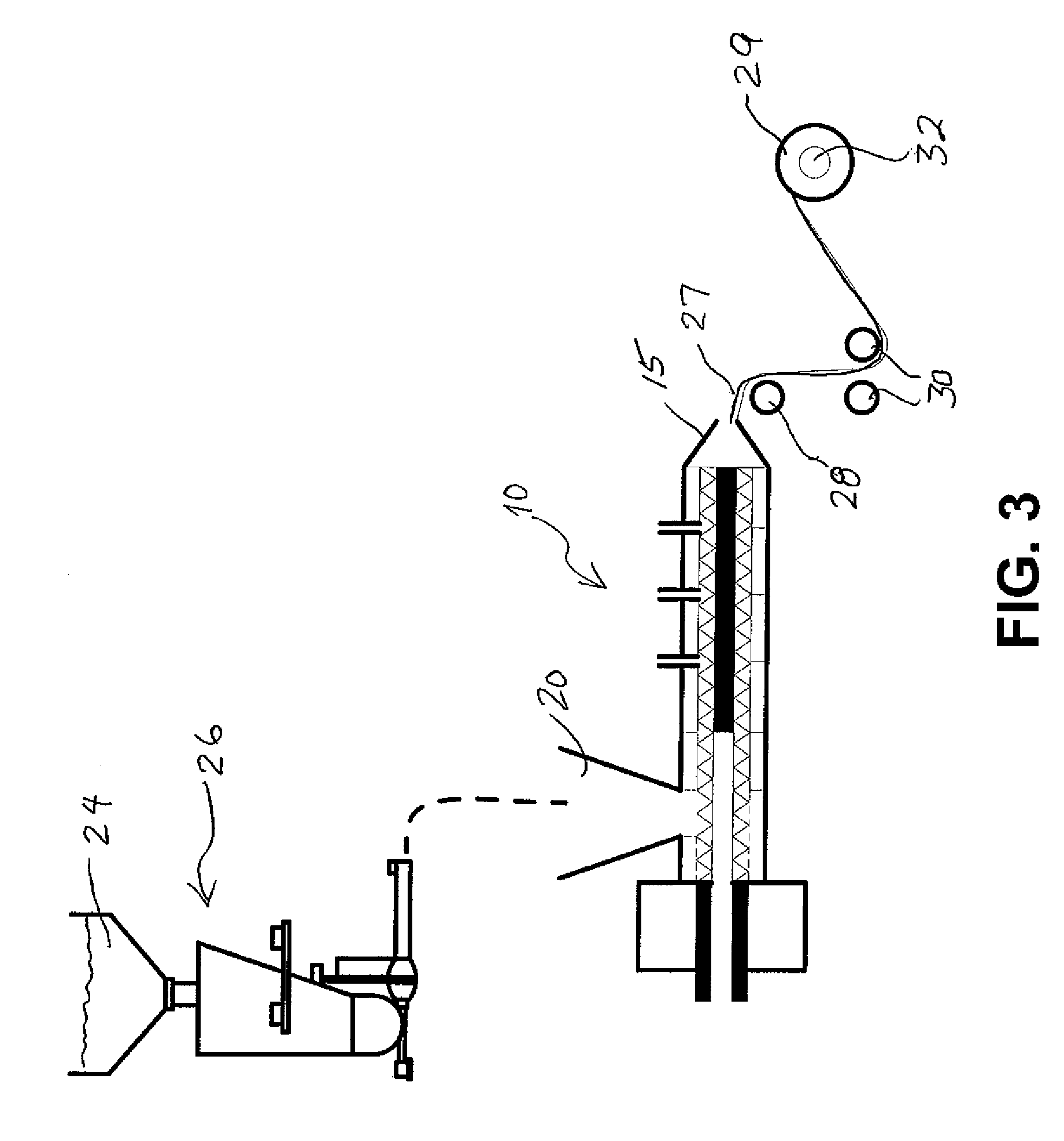
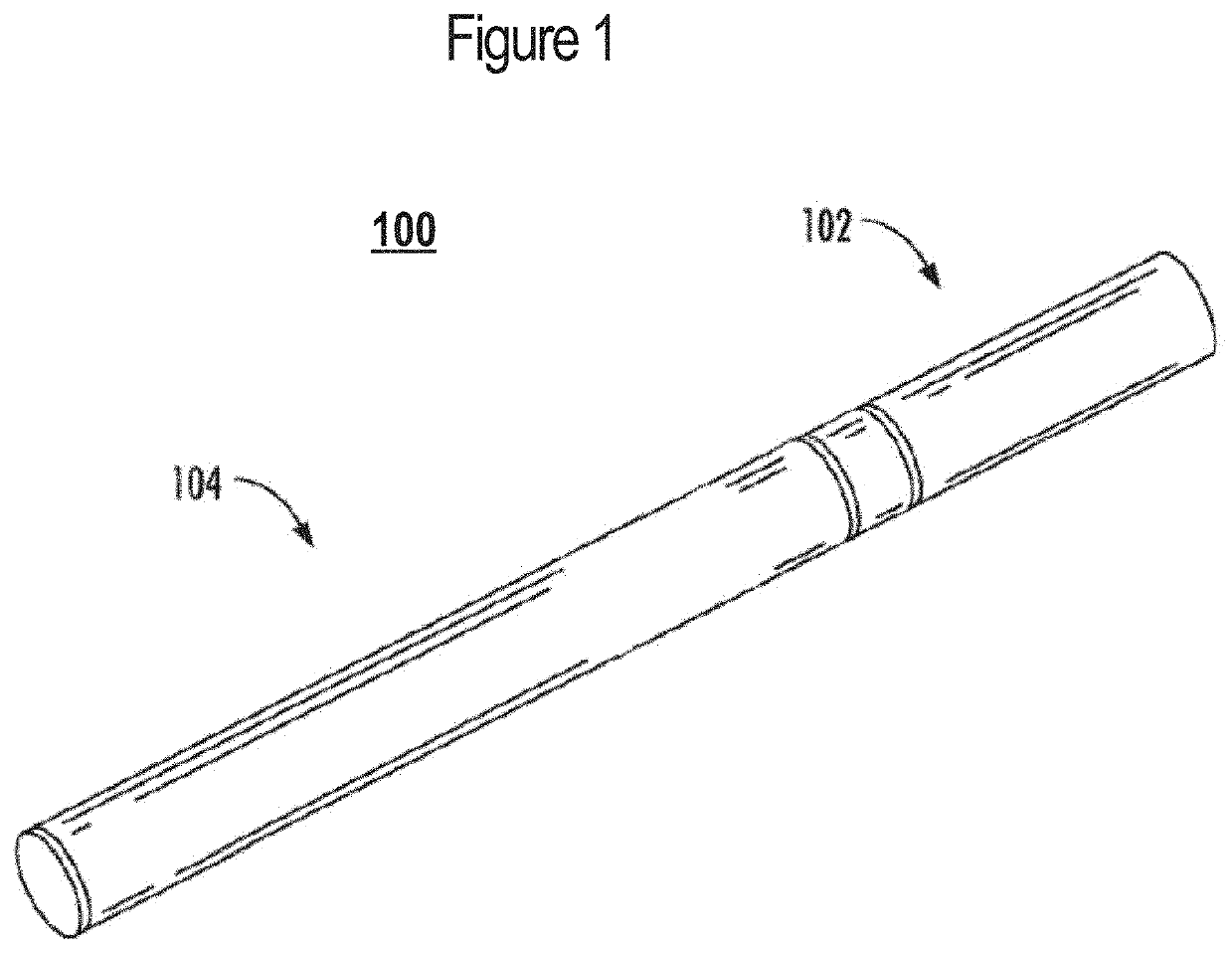
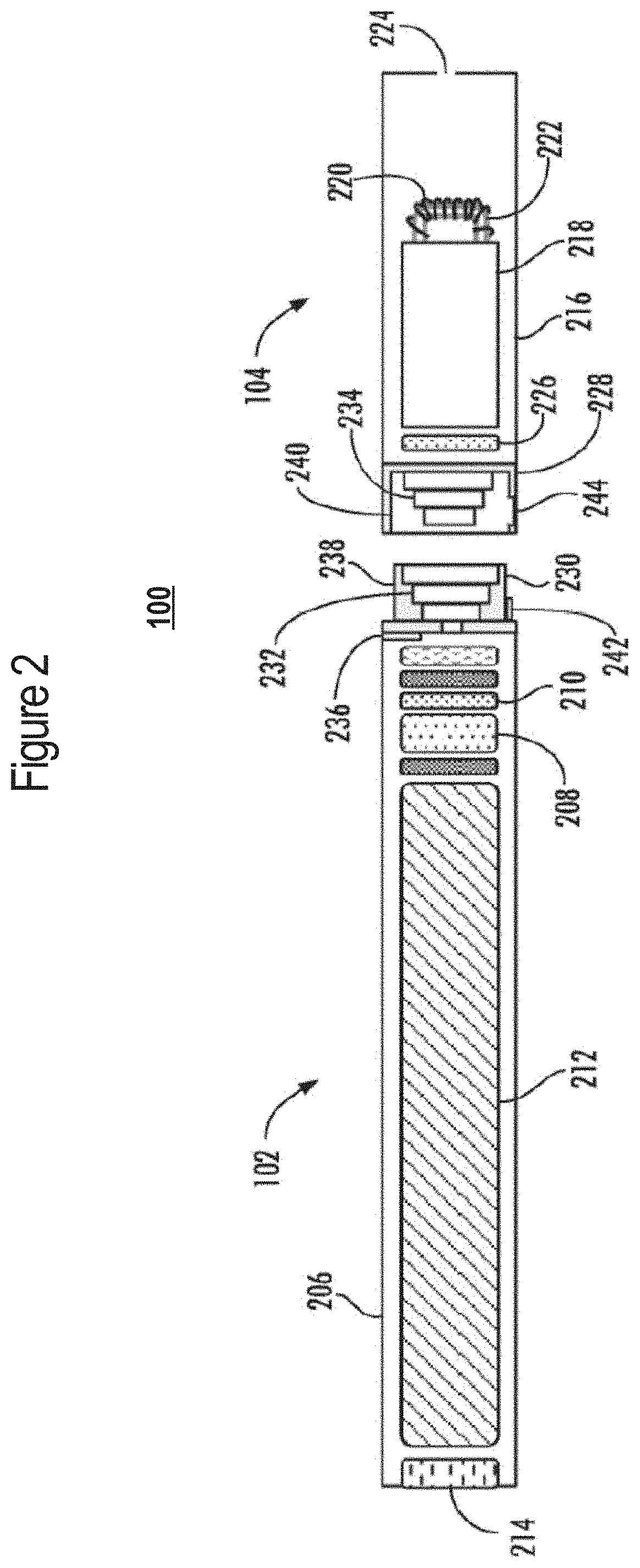
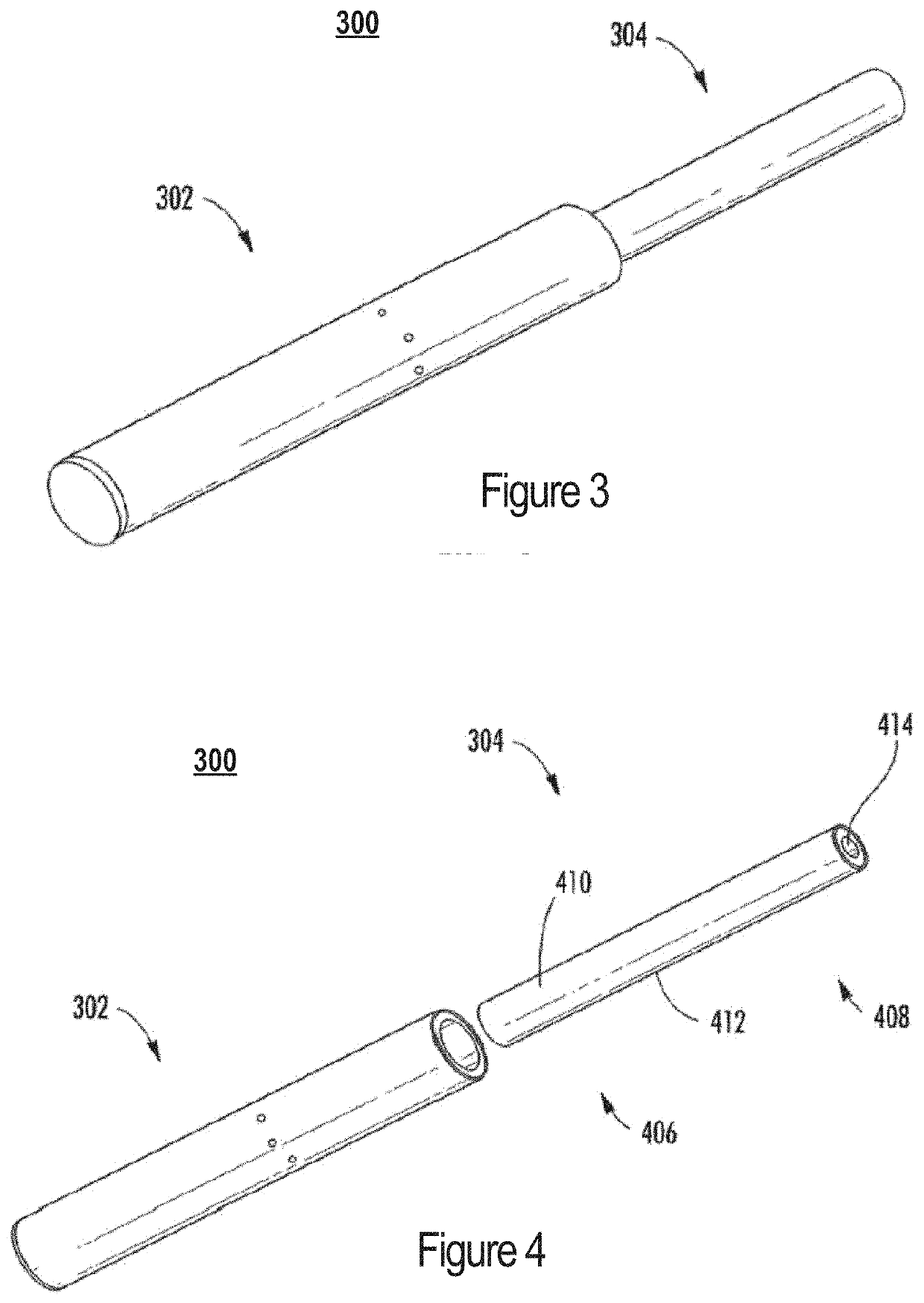
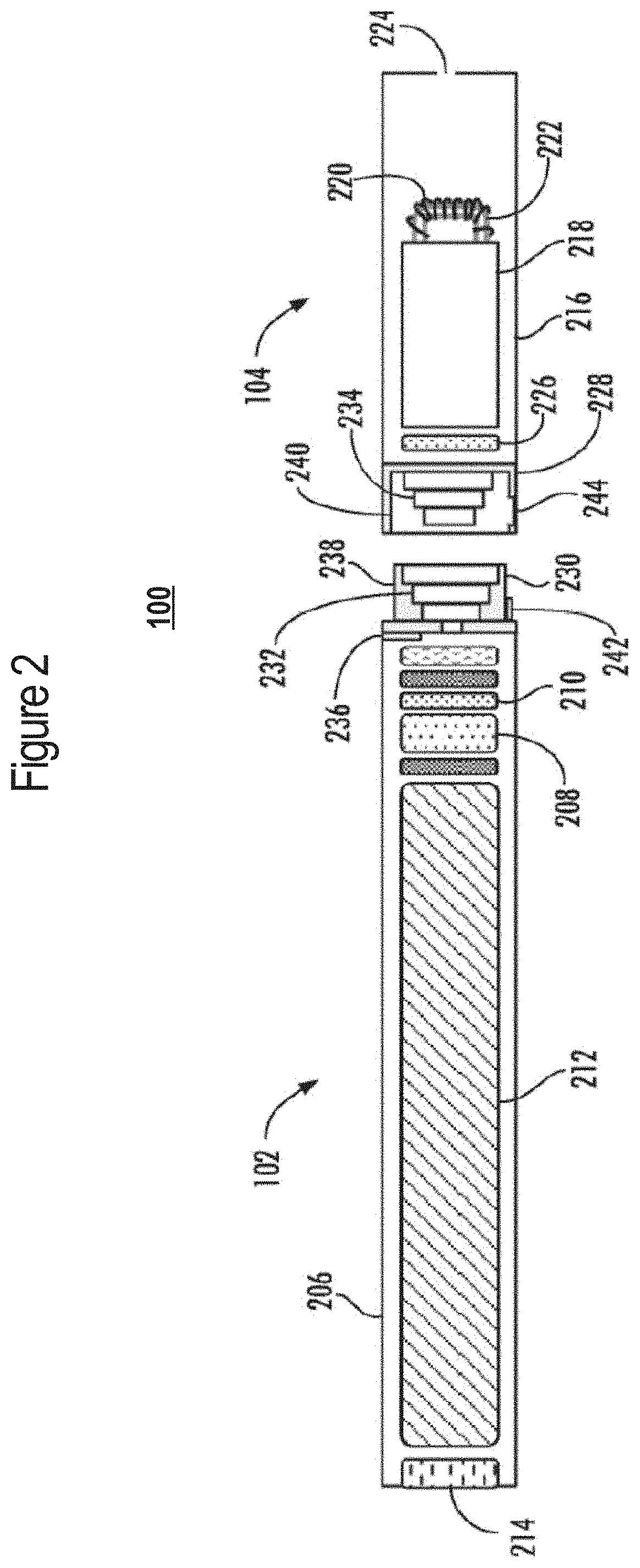
A nicotine delivery system is a product or device that delivers nicotine into your system as an alternative to smoking combustible cigarettes.
Buffered nicotine containing products
A pharmaceutical oral formulation for delivering nicotine in any form to a subject by transmucosal uptake in the oral cavity comprising nicotine in any form, wherein said oral formulation is buffered with at least one amino acid, preferably at least one endogenous amino acid. Also contemplated is a method for the oral delivery of nicotine in any form, a method for the reduction of the urge to smoke or use tobacco as well as methods for manufacturing the oral formulation, the use of said oral formulation for obtaining transmucosal uptake of nicotine in the oral cavity of a subject, and use of nicotine for the production of an oral formulation as per above for the treatment of a disease selected from the group consisting of tobacco or nicotine dependence, Alzheimer's disease, Crohn's disease, Parkinson's disease, Tourette's syndrome, ulcerous colitis and post-smoking-cessation weight control.
Owner:MCNEIL AB
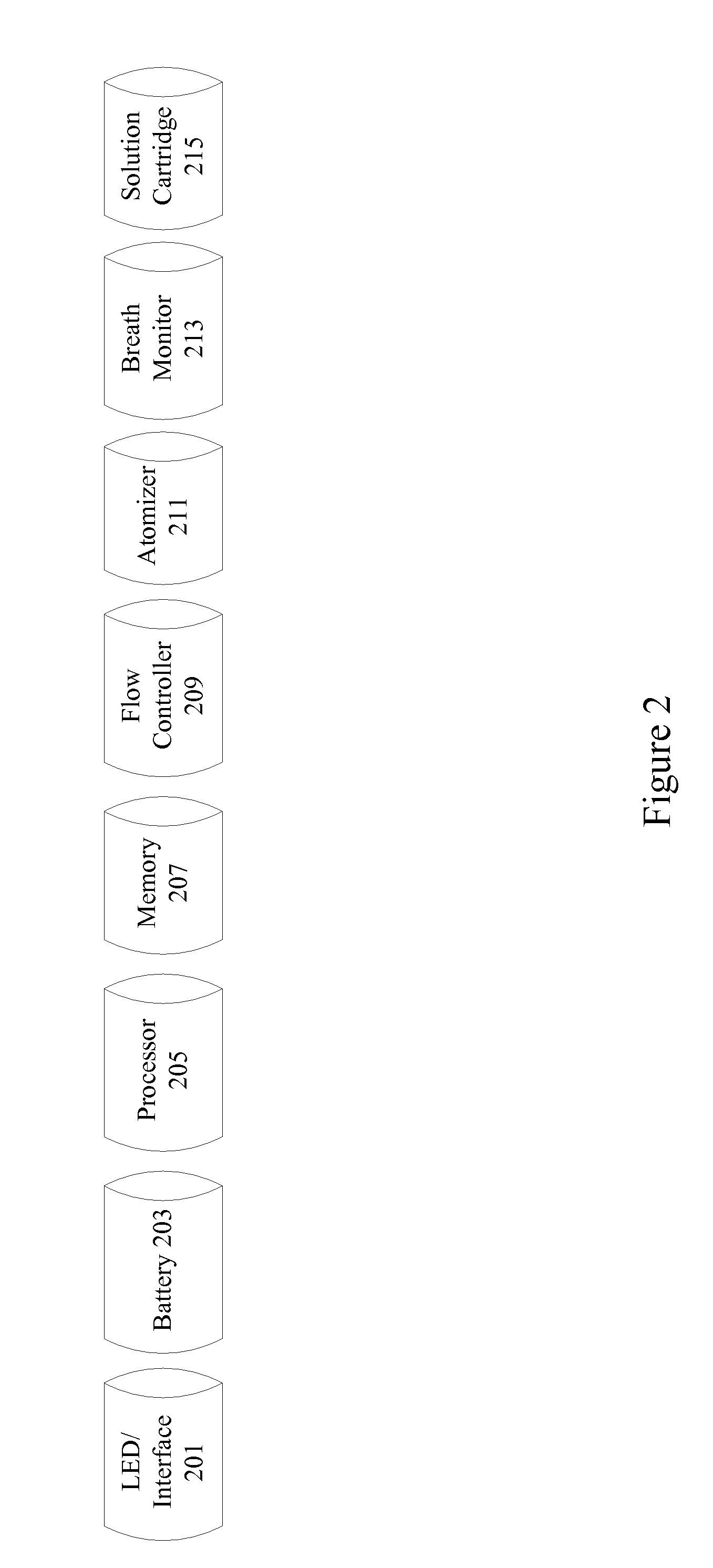
Methods and apparatus for nicotine delivery reduction
A nicotine delivery reduction system includes an electronic cigarette having a breath monitor and a flow controller. The breath monitor detects user breath characteristics such as breath duration, rate, depth, and strength and adjusts the amount of nicotine delivered to the user based on user breath characteristics. In particular examples, if the user is breathing with more urgency, additional nicotine is delivered to the user. The nicotine delivery reduction system also includes an interface to allow implementation of different reduction programs based on personal preferences and characteristics. If the user is breathing normally, the flow controller gradually reduces the level of nicotine delivered to the user. The nicotine delivery reduction system maintains user breath characteristics over time to allow reduction and possible elimination of nicotine dependence. Neuro-response data including electroencephalography (EEG) can be obtained and analyzed to determine the effectiveness of a nicotine reduction program.
Owner:THE NIELSEN COMPANY US A DELAWARE LIMITED LIABILITY
Two-stage transmucosal medicine delivery system for symptom relief
InactiveUS6893654B2Convenient and reliable and practicalReduce cravingsBiocidePowder deliveryOpiateInitial dose
A two-stage medicine delivery system provides an initial dose of medicine and a second dose of medicine. The initial and second doses are capable of achieving a rapid pharmacological effect and a prolonged pharmacological effect, respectively. The two-stage medicine delivery system preferably delivers a craving reduction substance, in which case, the rapid and prolonged pharmacological effects include a rapid and prolonged craving reduction. Preferably, the delivery system is a nicotine delivery system which is provided in chewing gum form or lozenge form and which provides the nicotine in a transmucosally absorbable form. The two-stage medicine delivery system preferably releases a buffering agent which increases a pH level in a user's mouth to facilitate absorption of the medicine when the delivery system is placed in the user's mouth. A method of making the medicine delivery system also is provided. The system and apparatus can be adapted to reduce cravings for alcohol, food, drugs (e.g., cocaine, opiates and the like) and tobacco products, especially tobacco products containing nicotine.
Owner:JSR NTI
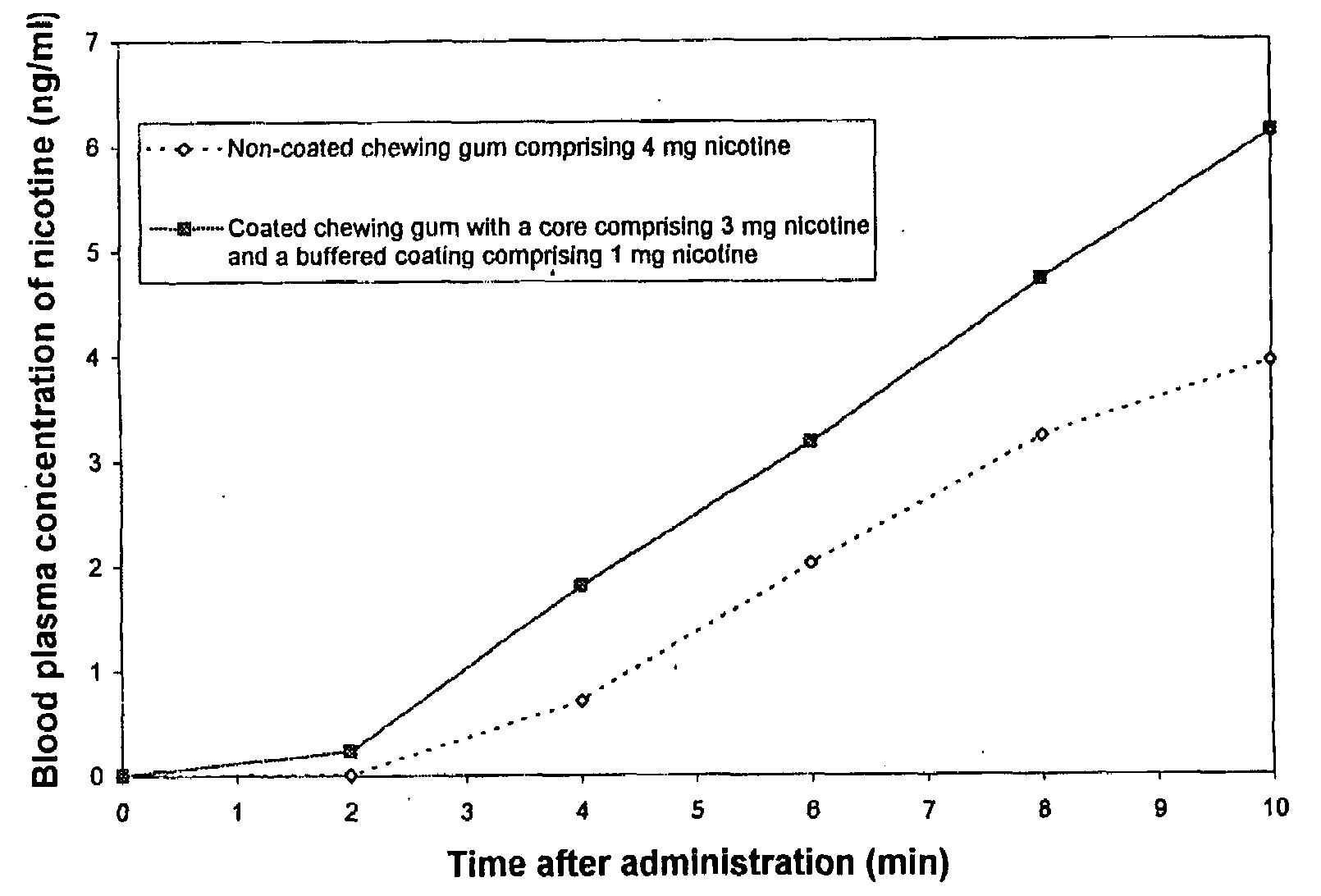
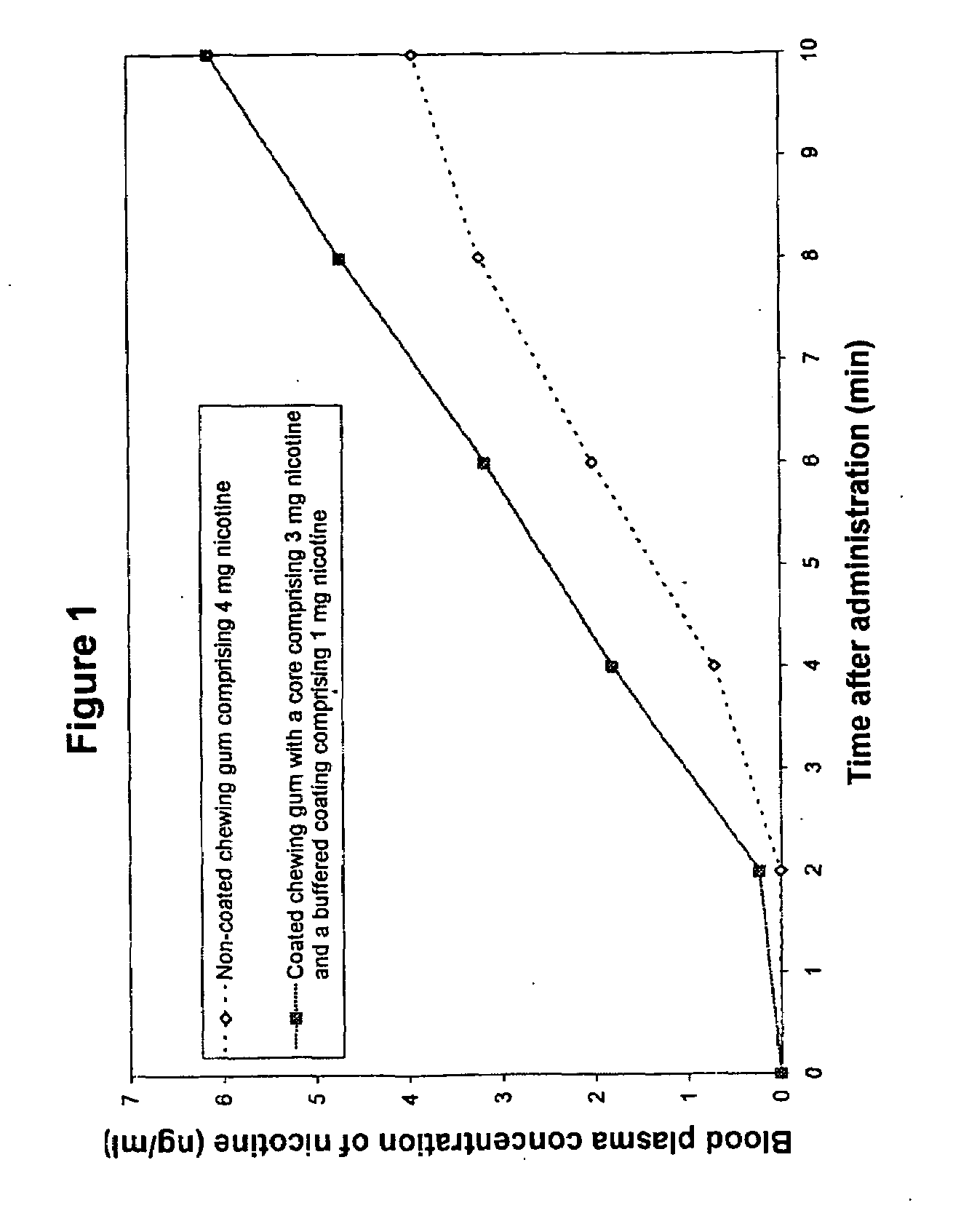
Buffered coated nicotine containing products
InactiveUS20080286341A1Rapid sufficient uptakePleasant tasteBiocideNervous disorderBuffering agentAmino acid
Coated oral dosage forms for the delivery of nicotine in any form to a subject by rapid intraoral delivery of nicotine comprising at least one core, nicotine in any form and / or a nicotine mimicking agent, at least one coating layer and optionally at least one or more other additives, wherein said at last one coating layer is buffered, whereby is used at least one amino acid as buffering agent. Also contemplated are a method for the delivery of nicotine in any form, a method for the reduction of the urge to smoke or use tobacco as well as a method for producing said coated product and use of the same for obtaining a rapid intraoral uptake of nicotine.
Owner:ANDERSSON SVEN BORJE +5
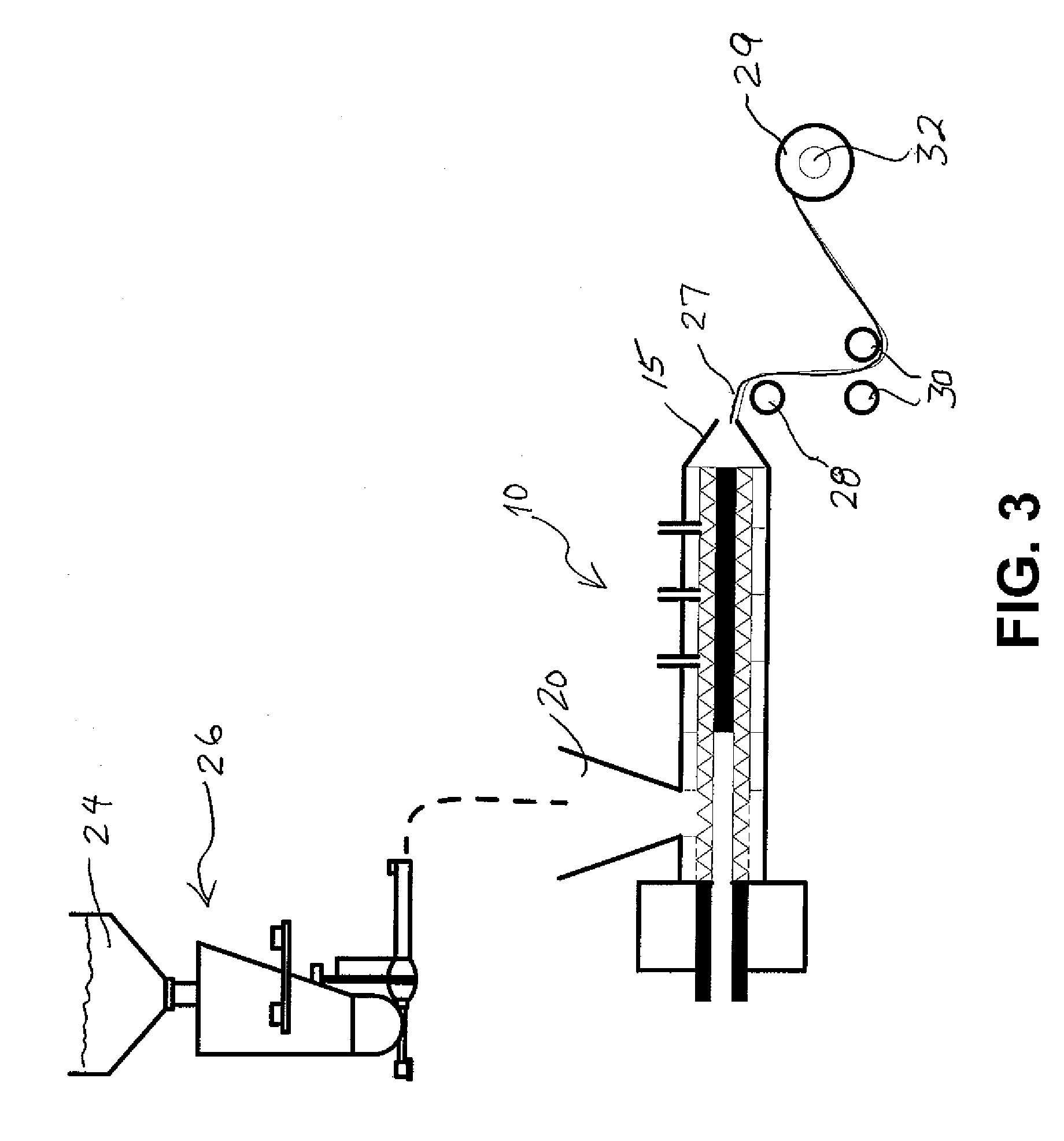
Method, composition and apparatus for functionalization of aerosols from non combustible smoking articles
ActiveUS20150374035A1Simple to fillImprove the preparation effectTobacco treatmentTobacco pipesNicotine deliveryAerosol
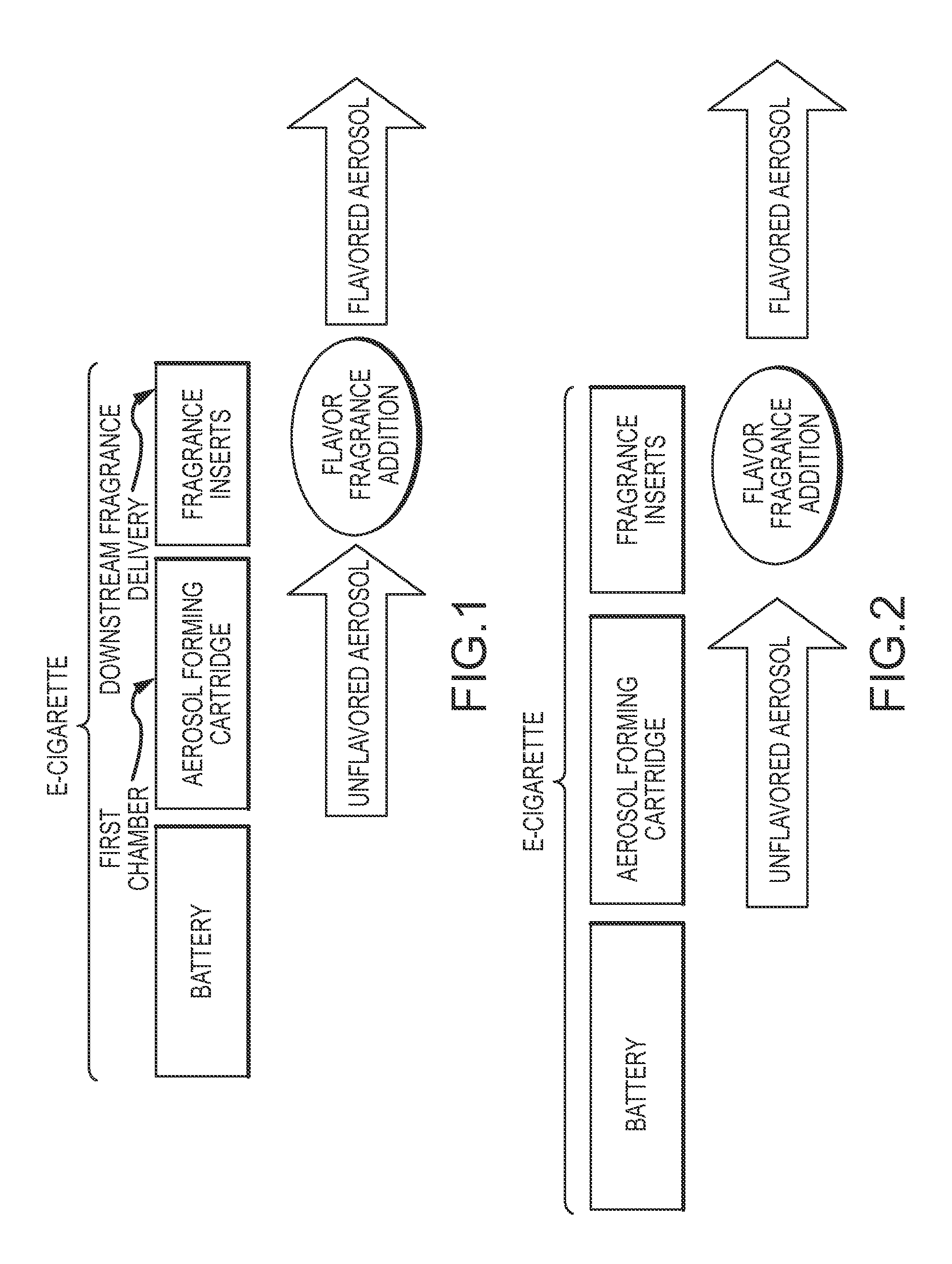
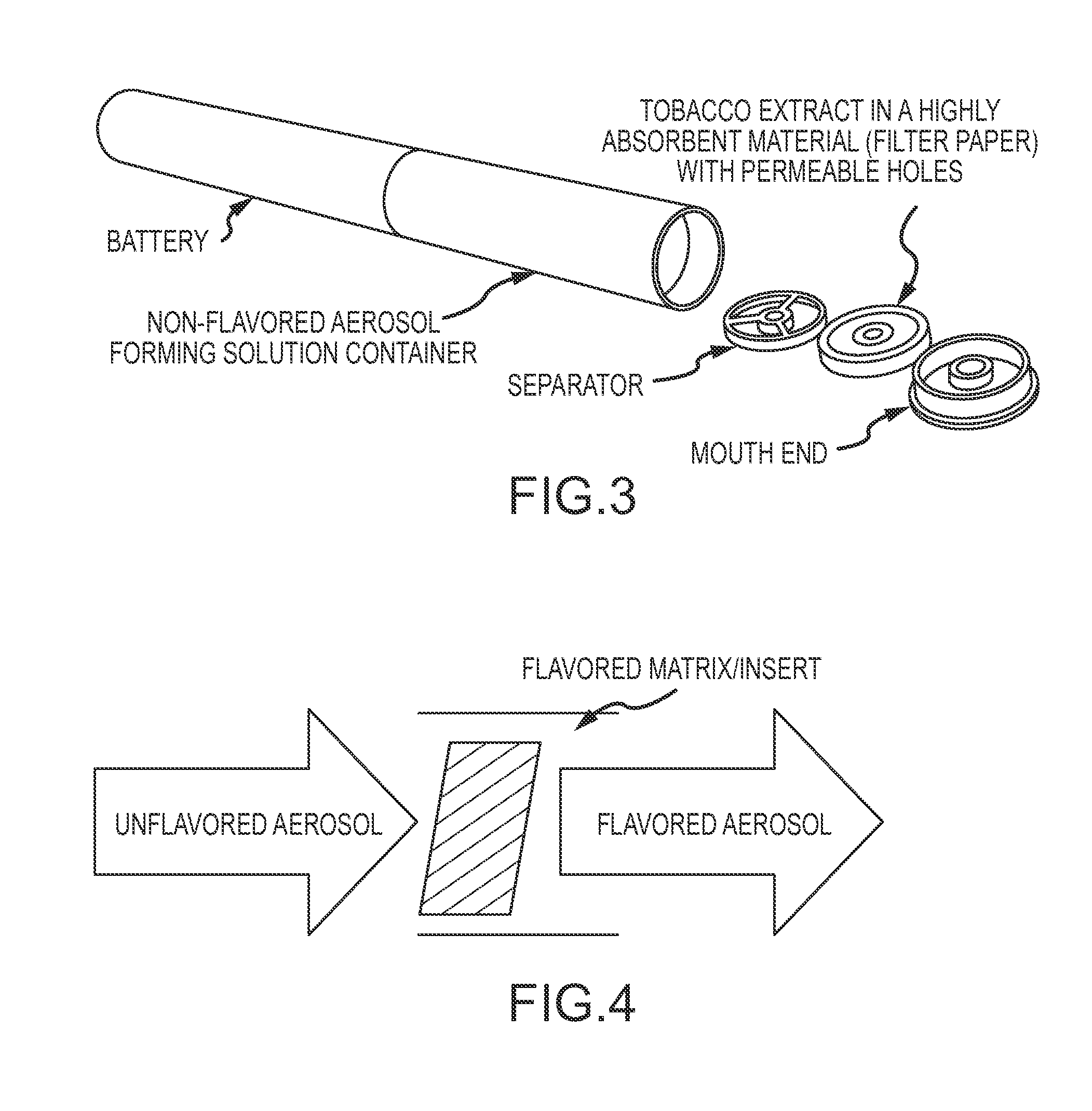
An apparatus and method for delivering an aerosol-forming composition and a separate functional composition for generating a functionalized aerosol vapor which emulates the organoleptic characteristics and properties of mainstream smoke experienced by smoking traditional tobacco-based smoking articles. The apparatus comprises an aerosol-forming liquid which is adapted to deliver aerosol-forming liquid to a heating device and a downstream chamber or zone containing an functional composition comprising one or more organoleptic components such as a taste, fragrance and / or nicotine delivery components. The method comprises generating an aerosol from an aerosol forming liquid and functionalizing the aerosol by subjecting the aerosol to a matrix for the purpose of transferring, delivering or imparting one or more organoleptic properties.
Owner:FONTEM HLDG 1
Smokeless Tobacco Product, Smokeless Tobacco Product in the Form of a Sheet, Extrudable Tobacco Composition, Method for Manufacturing a Smokeless Tobacco Product, Method for Delivering Super Bioavailable Nicotine Contained in Tobacco to a User, and Packaged Smokeless Tobacco Product Sheet
ActiveUS20090095313A1Sustained release of nicotine to the userTobacco preparationTobacco treatmentHot meltDissolution
A nonaqueous, extrudable composition includes at least one thermoplastic polymer in an amount of more than 20 wt % of the whole composition and tobacco. A smokeless tobacco product in the form of a sheet can be made by extruding or hot melt shaping a nonaqueous composition comprising at least one thermoplastic polymer and tobacco, the sheet being soluble in a user's mouth and resulting in sustained release of nicotine to the user. The sheet can be in a form that may be placed in the buccal cavity of, on the palate of or sublingually in the user, and have an average dissolution time of 5 to 50 minutes for delivering super bioavailable nicotine to the user.
Owner:PHILIP MORRIS PROD SA
New product and use and manufacture thereof
InactiveUS20070269386A1Improve buffering effectBiocideNervous disorderCrohn's diseaseNicotine dependence
A pharmaceutical oral formulation for delivering nicotine in any form to a subject by transmucosal uptake in the oral cavity comprising nicotine in any form, wherein said oral formulation is buffered with at least trometamol. Also contemplated is a method for the oral delivery of nicotine in any form, a method for the reduction of the urge to smoke or use tobacco as well as methods for manufacturing the oral formulation, the use of the oral formulation for obtaining transmucosal uptake of nicotine in the oral cavity of a subject, and use of nicotine for the production of an oral formulation for the treatment of a disease selected from the group consisting of tobacco or nicotine dependence, Alzheimer's disease, Crohn's disease, Parkinson's disease, Tourette's syndrome, ulcerous colitis and post-smoking-cessation weight control.
Owner:MCNEIL AB
New product and use and manufacture thereof
InactiveUS20070269492A1Promote withdrawalRapid satisfactionOrganic active ingredientsNervous disorderBuffering agentDosage form
Coated oral dosage forms for the delivery of nicotine in any form to a subject by rapid intraoral delivery of nicotine comprising at least one core, nicotine in any form and / or a nicotine mimicking agent, at least one coating layer and optionally at least one or more other additives, wherein said at last one coating layer is buffered, whereby is used at least trometamol as buffering agent. Also contemplated is a method for the delivery of nicotine in any form, a method for the reduction of the urge to smoke or use tobacco as well as a method for producing said coated product and the use of the same for obtaining a rapid intraoral uptake of nicotine.
Owner:MCNEIL PPC INC
Medicated toothpick
InactiveUS20050058609A1Efficient deliveryEliminate effectiveCosmetic preparationsGum massageMedicineToothpick
A medicated toothpick for administering a selected dosage of medication including a toothpick shaped device impregnated with a selected quantity of medication. The medicated toothpick is particularly useful for the delivery of nicotine and provides a physical and mental nicotine delivery system that may effectively be substituted for smoking.
Owner:NAZERI ALIREZA
Method of manufacturing a nicotine delivery product
A method of manufacturing a nicotine delivery product comprising nicotine and a cation exchange resin. More precisely, to a method for preparing a nicotine delivery product said method comprising (a) mixing nicotine, a cation exchange resin, an organic polyol and water to form a mixture wherein the total amount of water is from 26 to 45% by weight of the total mixture, and (b) removing water from the mixture to produce said nicotine delivery product.
Owner:FERTIN PHARMA AS
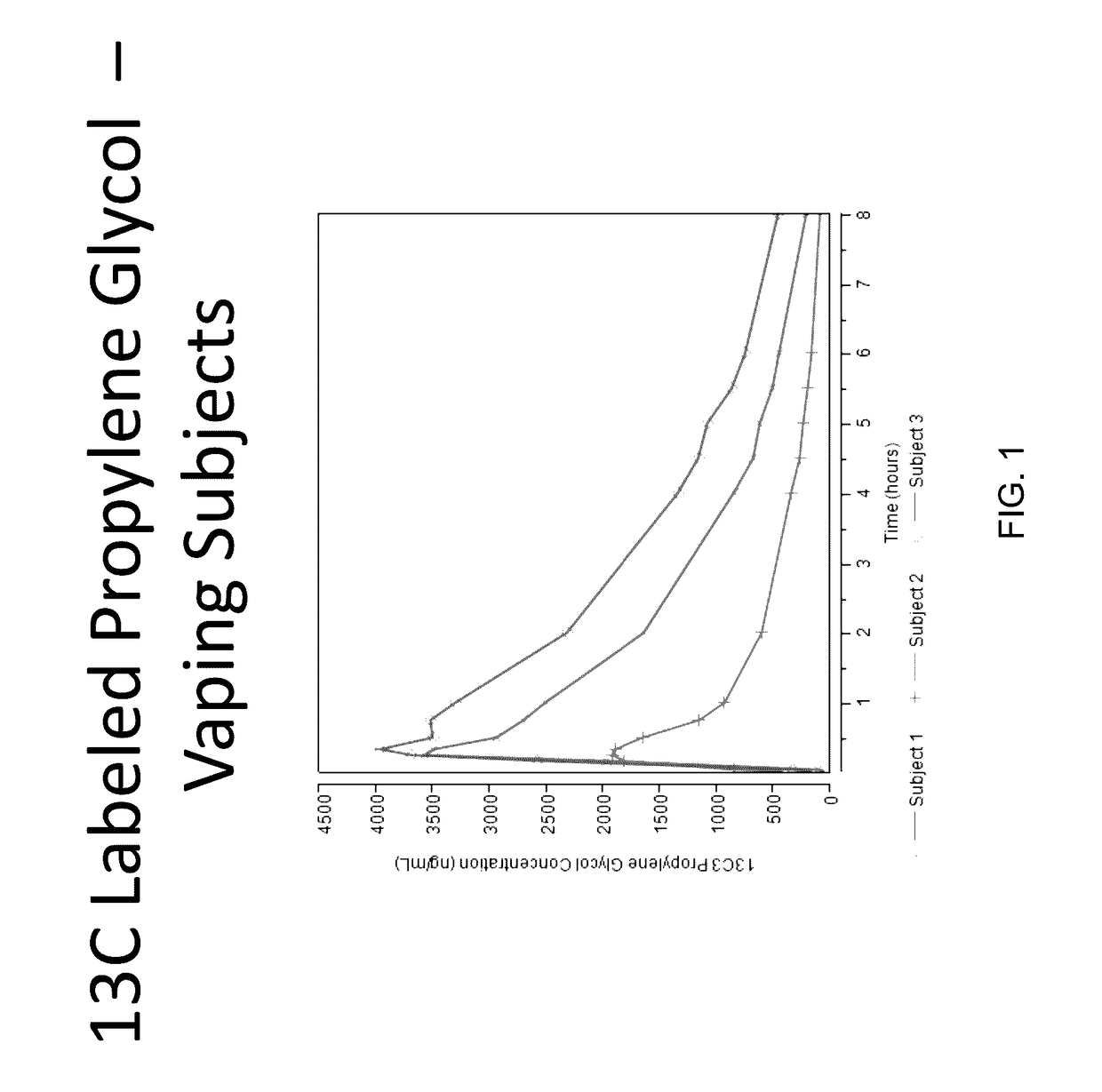
Isotopically-labeled solvents and the use of same in testing e-cigarettes
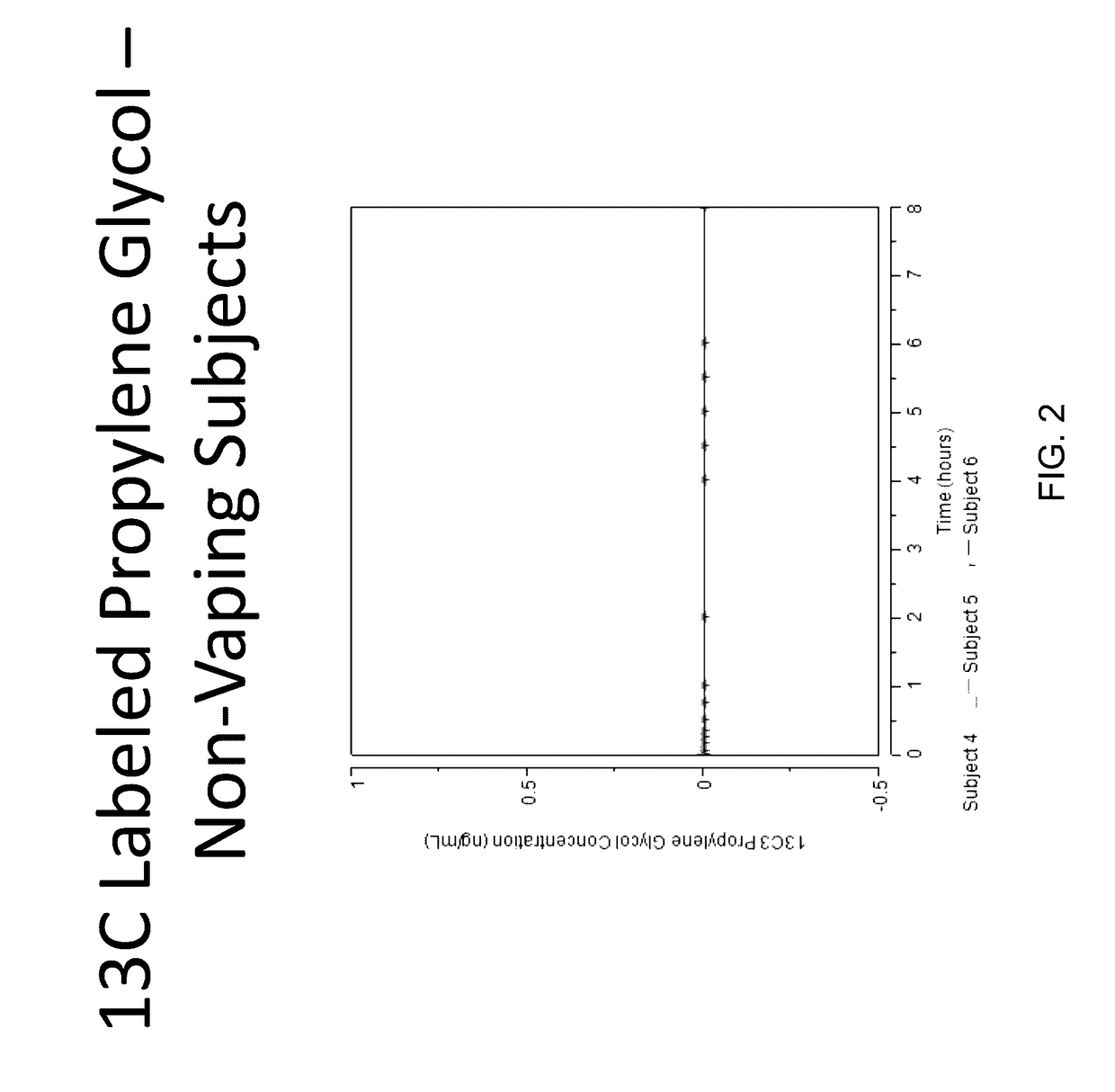
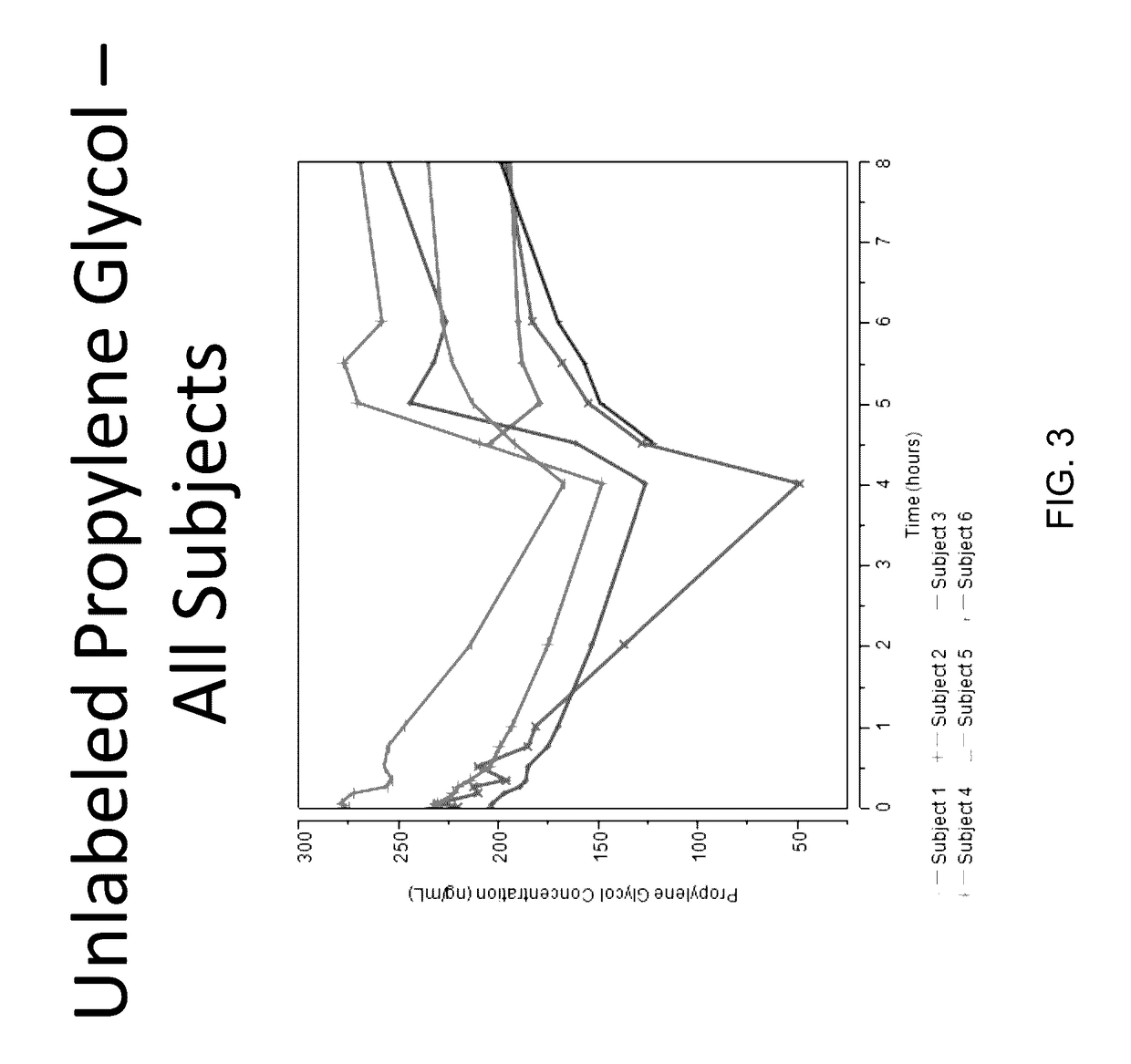
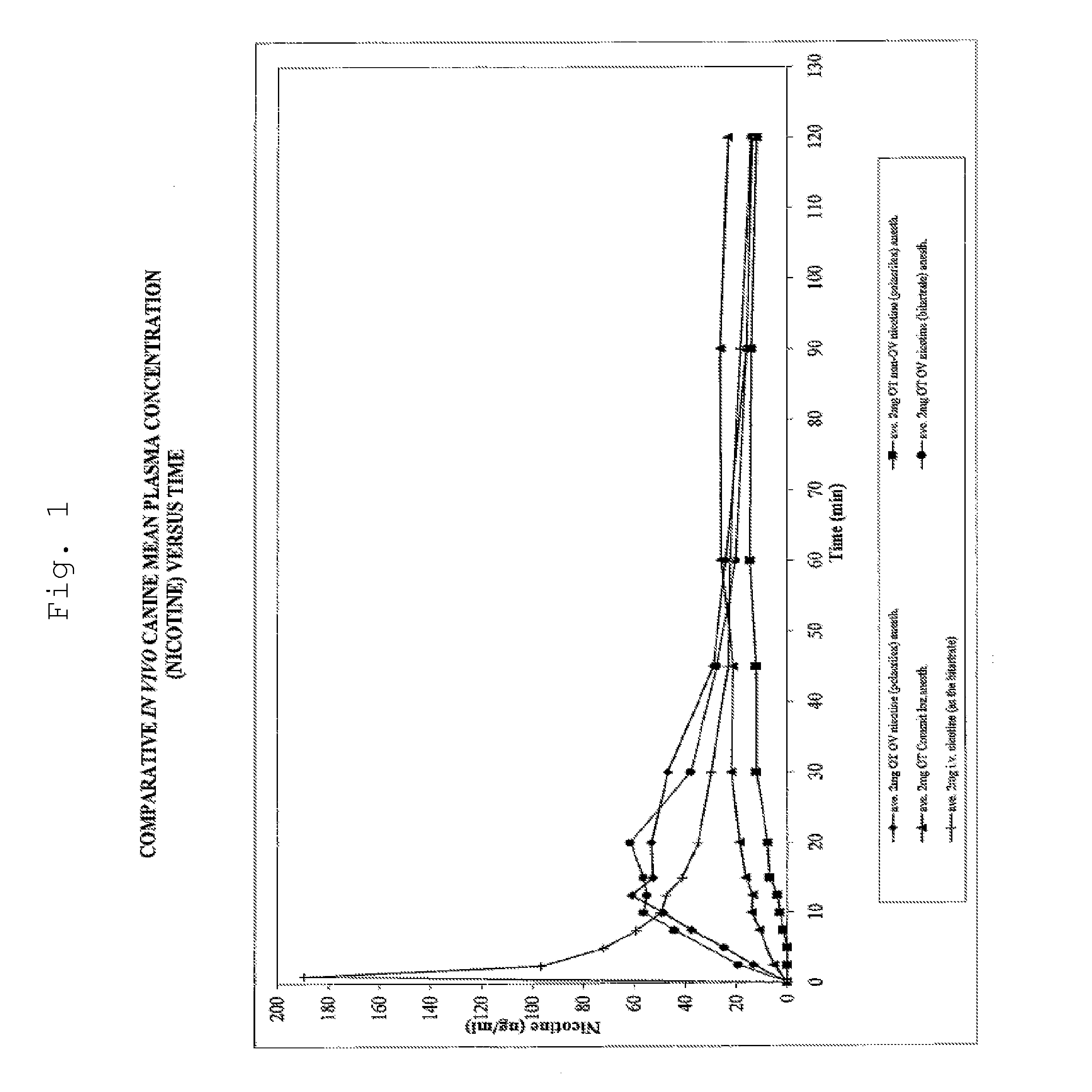
Isotopically-labeled species of propylene glycol and glycerol are provided as solvents for use in electronic nicotine delivery systems (e.g., as disposed in a cartridge in an e-cigarette). The isotopically-labeled species are distinguishable from the non-isotopically-labeled species (e.g., by mass spectrometry). Thus, methods are provided for the measurement of the quantity of solvent or of a solvent heating by-product (e.g., formaldehyde, acetaldehyde), or of a metabolite of the drug, delivered to a user by analysis of blood samples taken subsequent to dosing by use of the electronic nicotine delivery system. An example of an isotopically-labeled species of propylene glycol for use in such measurement methods is [13C]3H8O2. A clinical study of e-cigarettes loaded with this isotopically-labeled species resulted in the following blood plasma concentration profiles in “vaping” subjects.
Owner:CELERION INC
Smokeless tobacco product
InactiveUS20100242978A1Sustained release of nicotine to the userTobacco preparationNervous disorderMedicineDissolution
A nonaqueous, extrudable composition includes at least one thermoplastic polymer in an amount of more than 20 wt % of the whole composition and tobacco. A smokeless tobacco product in the form of a sheet can be made by extruding or hot melt shaping a nonaqueous composition comprising at least one thermoplastic polymer and tobacco, the sheet being soluble in a user's mouth and resulting in sustained release of nicotine to the user. The sheet can be in a form that may be placed in the buccal cavity of, on the palate of or sublingually in the user, and have an average dissolution time of 5 to 50 minutes for delivering super bioavailable nicotine to the user.
Owner:PHILIP MORRIS PROD SA
Nicotine Delivery Product and Method for Producing It
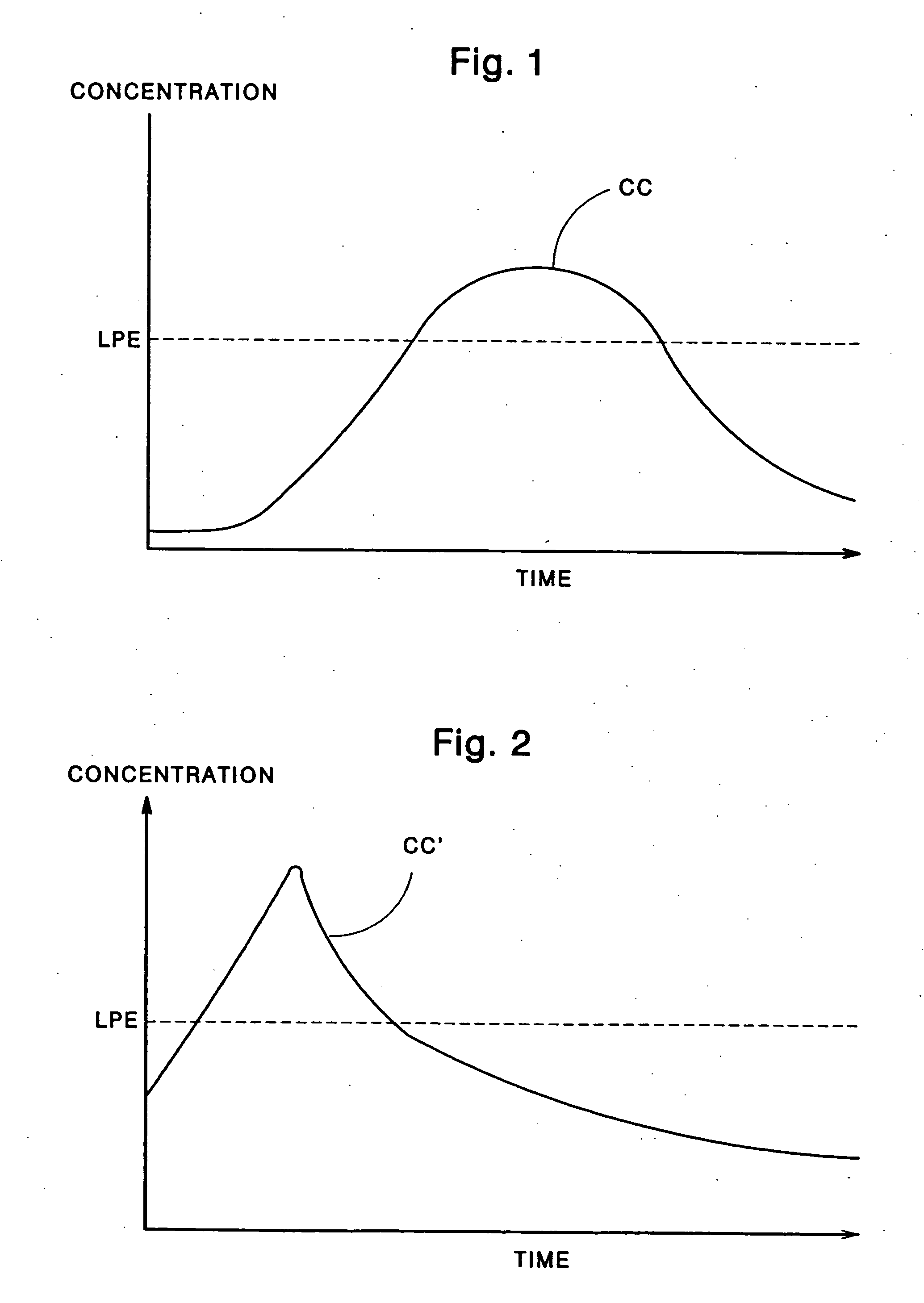
ActiveUS20080038209A1Same loading capacityIncrease release rateBiocideNervous disorderPolyolChewing gum
A nicotine delivery product comprising the reaction product of a nicotine / cation exchange resin complex and an organic polyol; and a method for preparing it comprising (a) mixing an aqueous suspension of a nicotine / cation exchange resin complex with an organic polyol or an aqueous solution thereof, and (b) removing water from the mixture to produce said nicotine delivery product. The nicotine delivery product has a nicotine release rate of at least 80% over a 10 minute period. It is particularly suited for use in smoking substitution devices delivering nicotine such as chewing gum, patches, lozenges, melting tablets and tablets for chewing.
Owner:FERTIN PHARMA AS
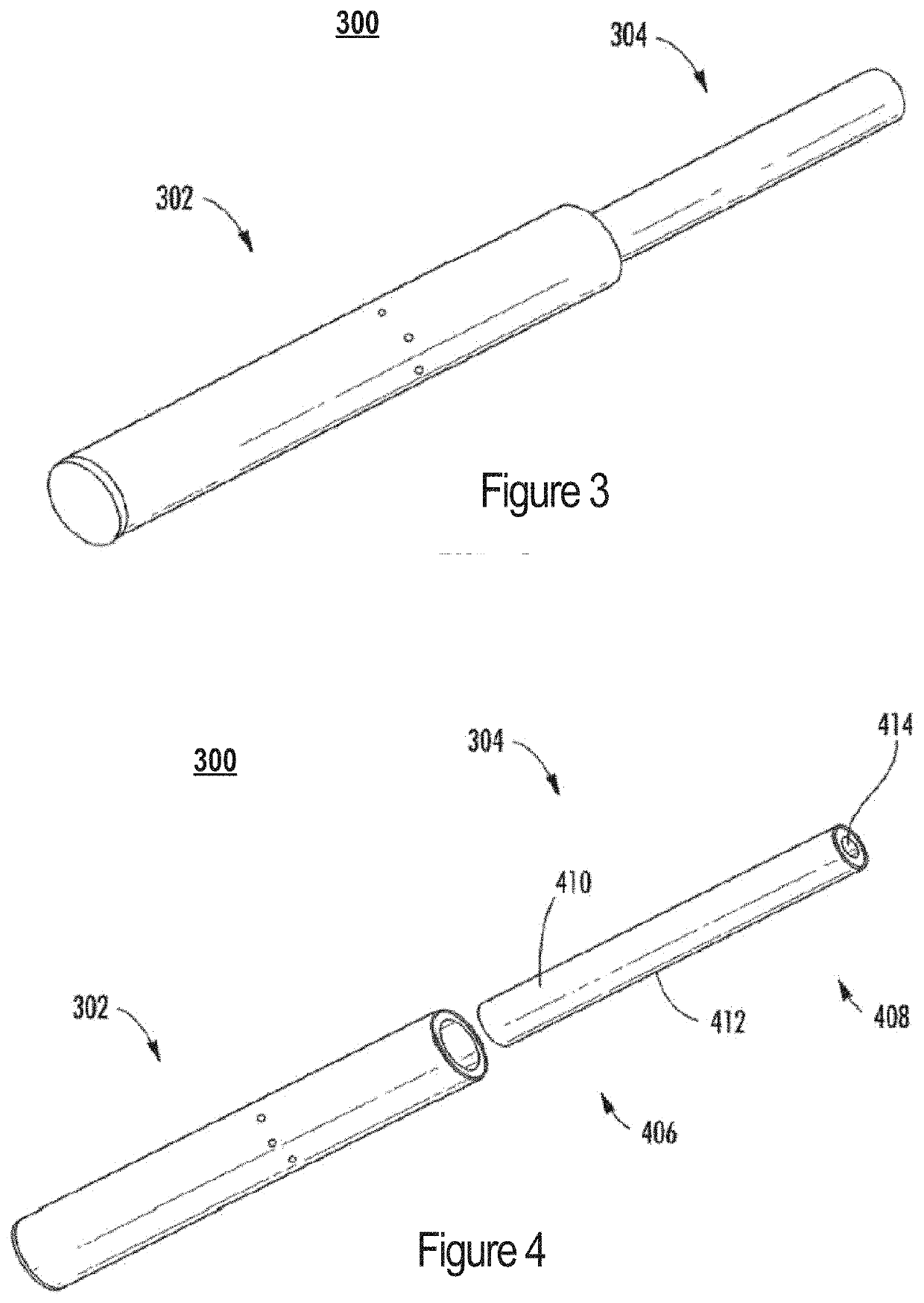
System and process for tobaccoless nicotine delivery
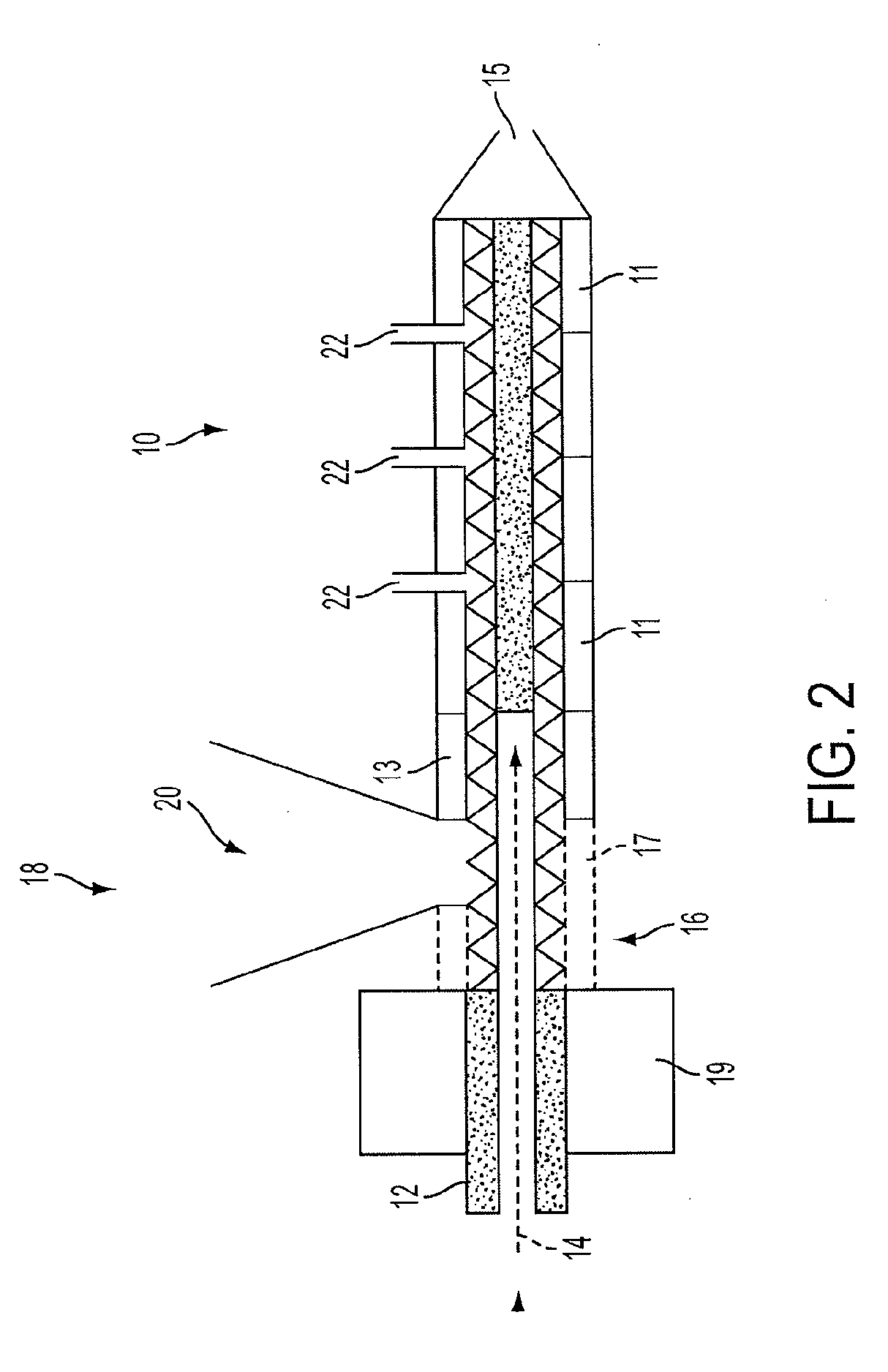
A tobaccoless nicotine delivery device permits delivery of nicotine in a manner similar to a cigarette. The device may include an impermeable outer layer surrounding a micro-encapsulated nicotine layer that includes a measured amount of nicotine in a form that allows nicotine vapor to be released upon rupture of the micro-encapsulation. A permeable membrane may be disposed adjacent to the micro-encapsulated nicotine layer that allows pressure to be applied to the micro-encapsulated nicotine layer while permitting permeation of released nicotine into a central channel for inhalation by a user. A mouthpiece may be disposed at an open end of the central channel in order to provide air resistance to simulate the inhalation effect of a cigarette.
Owner:UMAGINATION LABS
Intraoral delivery of nicotine for smoking cessation
InactiveUS20070298090A1Promote absorptionIncrease success rateBiocideNervous disorderMucoadhesionSmoking cessation
Dosage forms of a nicotine delivery system are disclosed in which a mucoadhesive film, made up of one or more non-microbial hydrocolloid(s) and an effective dose of nicotine, dissolves when applied intraorally to release the nicotine which is absorbed through the oramucosac and directly reaches systemic circulation. Methods for preparing various versions of the dosage forms are disclosed. Methods to assist smoking cessation or provide substitutes for smoking by administrating the dosage form are also provided.
Owner:THALLIUM HLDG CO LLC
Two-stage transmucosal medicine delivery system for symptom relief
InactiveUS20050214229A1Convenient and reliable and practicalReduce cravingsBiocideDrug compositionsOpiateInitial dose
Owner:JSR NTI
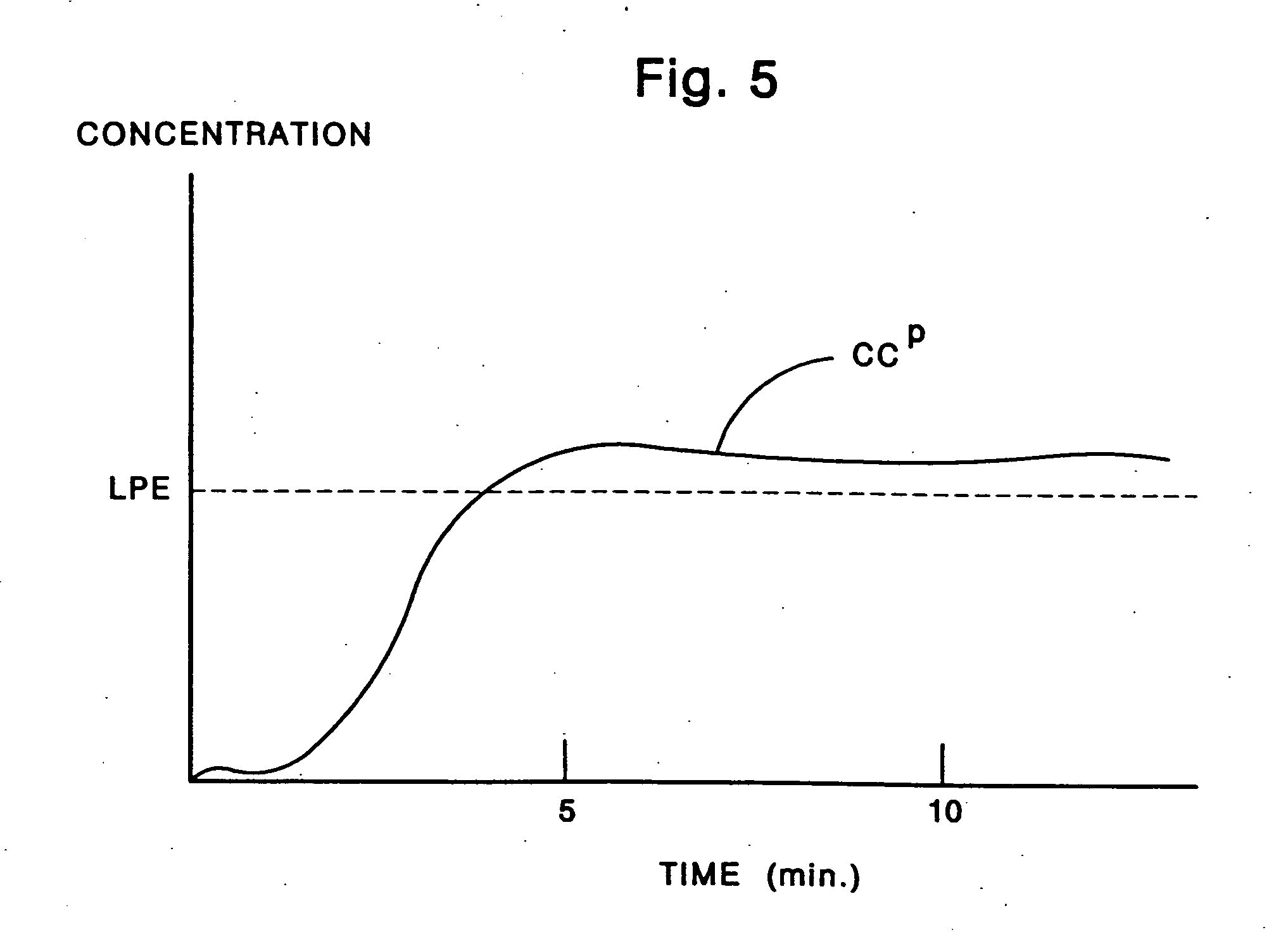
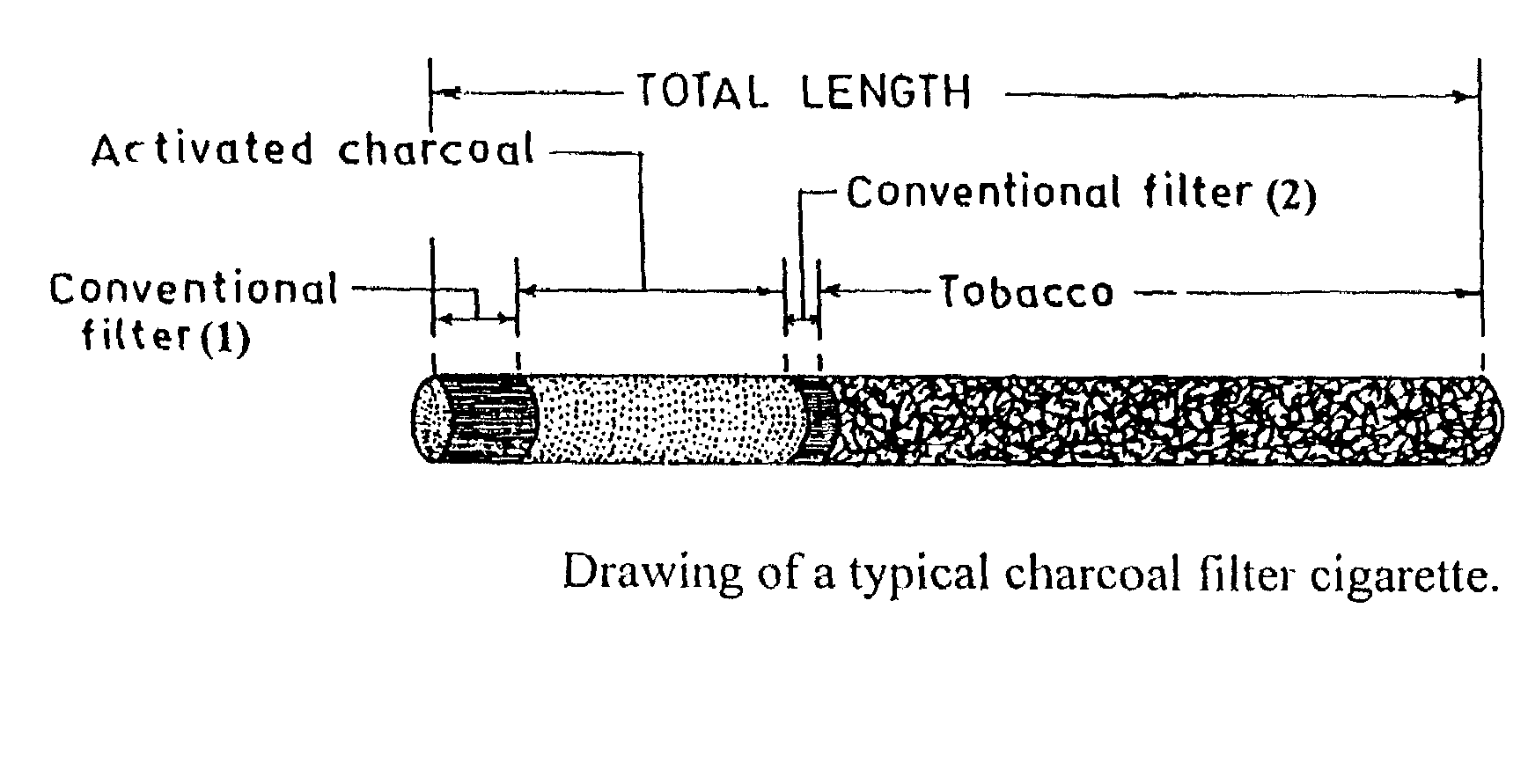
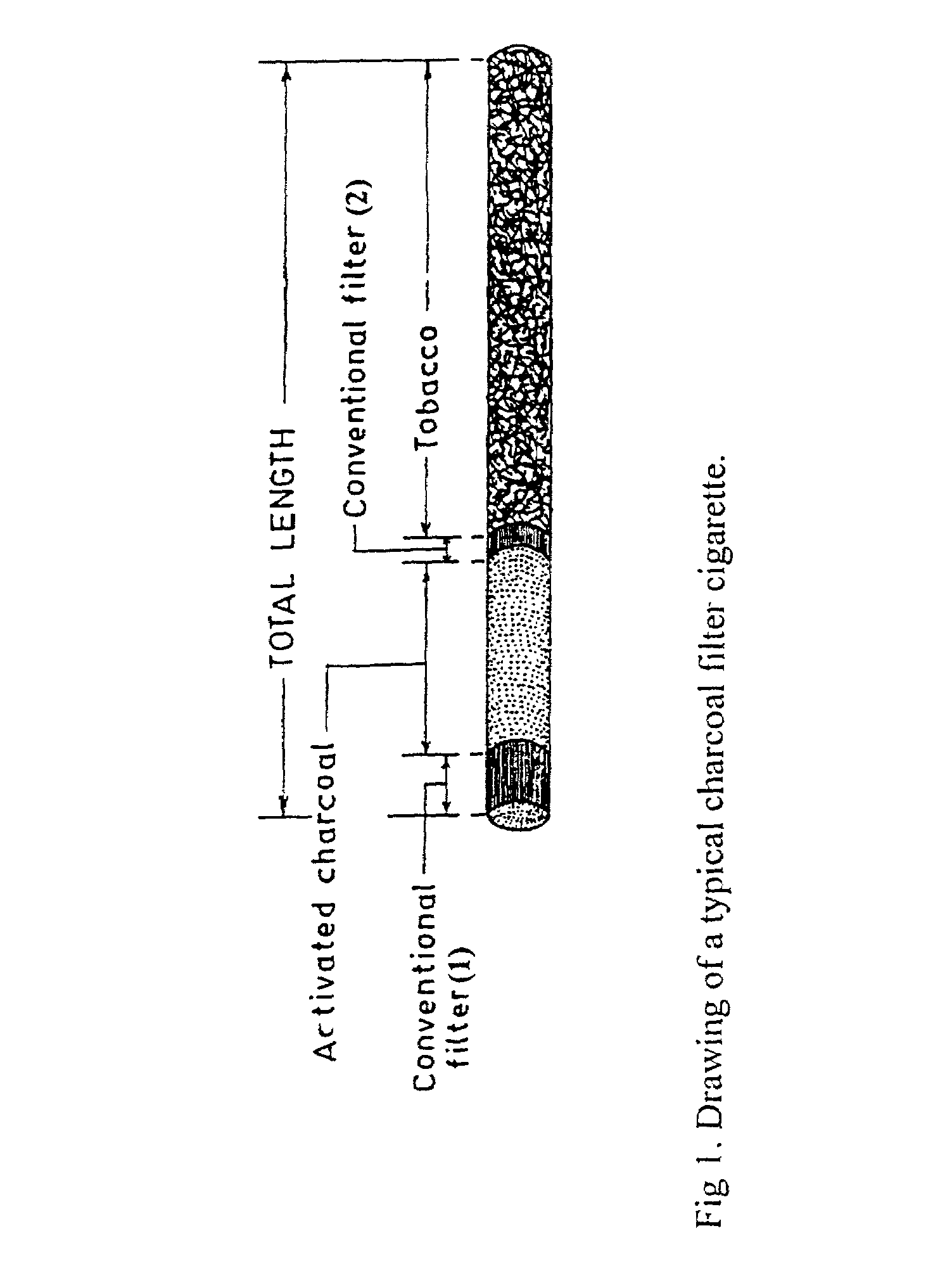
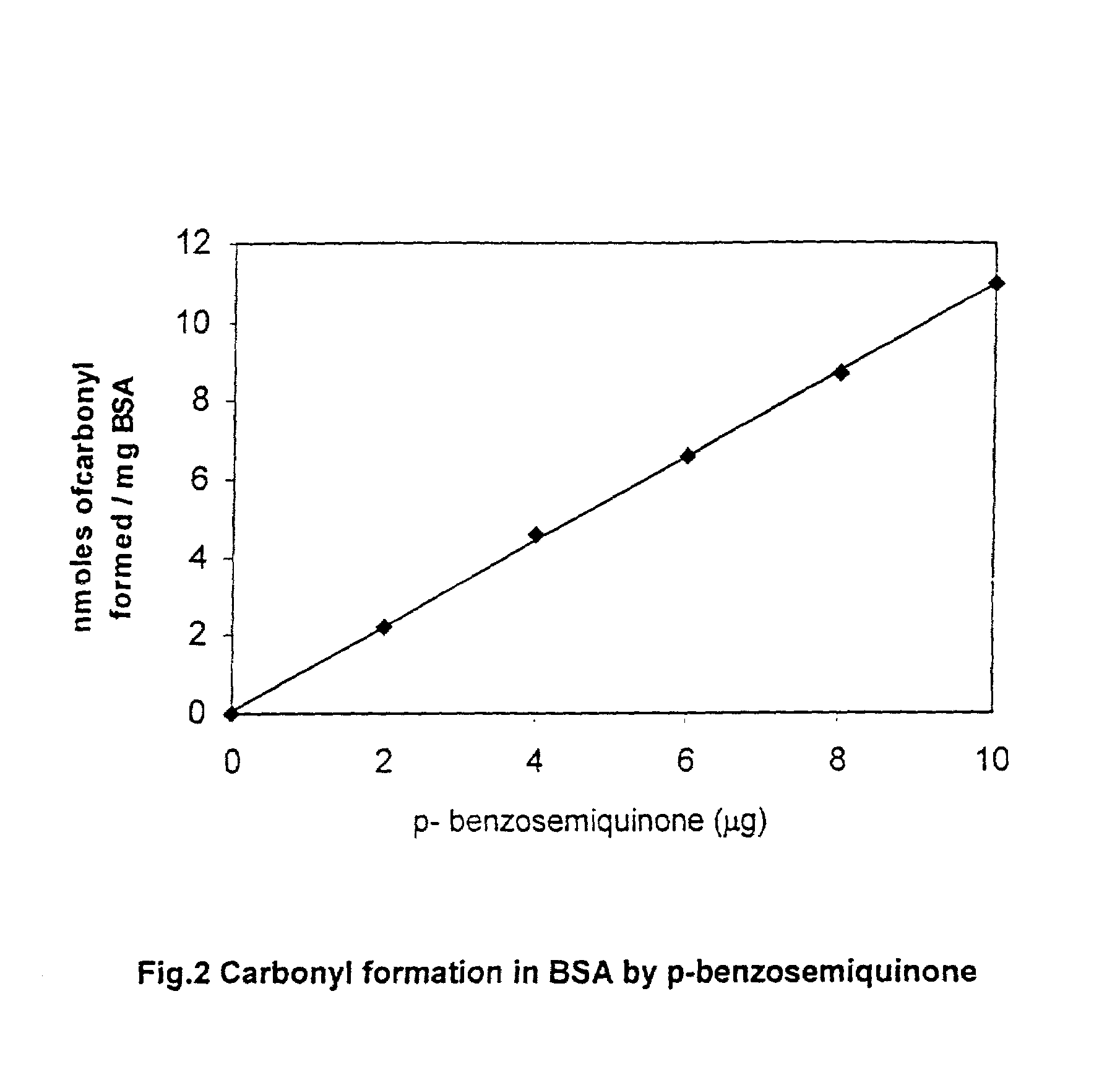
Activated charcoal filter for effectively reducing p-benzosemiquinone from the mainstream cigarette smoke
InactiveUS7025067B2Affecting flavourAffecting tasteTobacco treatmentTobacco smoke filtersActivated carbon filtrationNitric oxide
A filter for tobacco smoke inhaling / generating / producing device, comprising stipulated amounts of specific grain sizes or combination of grain sizes of activated charcoal for effectively reducing from the mainstream smoke the level of p-benzosemiquinone (p-BSQ), a relatively stable highly reactive major harmful oxidant, without significantly affecting the flavor and taste of the smoke while providing comfortable mouthful of smoke and nicotine delivery, so that the charcoal filter cigarettes becomes potentially less hazardous safer cigarettes and may be acceptable to the smokers with marked reduction in health risk; the charcoal filters also effectively reduce the level of nitric oxide and tar from the mainstream smoke.
Owner:COUNCIL OF SCI & IND RES
Decentralized identity storage for tobacco products
PendingUS20200342507A1Enhanced identity securityProvide mechanismCryptography processingCoin-freed apparatus detailsInternet privacyEngineering
Identity information can be stored in a decentralized structure. The identity information may include age verification information for the purchase or operation of certain tobacco products, such as an electronic nicotine delivery systems (“ENDS”) device, which may include aerosol delivery devices. The tobacco products or devices may have an age verification requirement or other identity requirements needed to authenticate a user and that information must be stored. A decentralized structure for storing the identity information may improve the security of that identity information while also providing a mechanism for accessing the information for verification or authentication purposes.
Owner:RAI STRATEGIC HLDG INC
Authentication and age verification for an aerosol delivery device
ActiveUS20200315259A1Circuit authenticationTransducer detailsAerosol deliveryIntensive care medicine
A charger for an electronic nicotine delivery systems (“ENDS”) device, which may include aerosol delivery devices provides functionality for authentication, including age verification. Such devices may be restricted based on age or other factors that require some form of authentication, verification, and / or identification to satisfy the restriction. The accessory or charger may provide or connect with a verification system for confirming an age of a user. If the authentication or verification is not satisfied, the charger or accessory will not charge the device, rendering it unusable.
Owner:RAI STRATEGIC HLDG INC
Smokeless tobacco product, smokeless tobacco product in the form of a sheet, extrudable tobacco composition, method for manufacturing a smokeless tobacco product, method for delivering super bioavailable nicotine contained in tobacco to a user, and packaged smokeless tobacco product sheet
ActiveUS9125434B2Sustained release of nicotine to the userTobacco preparationTobacco treatmentHot meltDissolution
A nonaqueous, extrudable composition includes at least one thermoplastic polymer in an amount of more than 20 wt % of the whole composition and tobacco. A smokeless tobacco product in the form of a sheet can be made by extruding or hot melt shaping a nonaqueous composition comprising at least one thermoplastic polymer and tobacco, the sheet being soluble in a user's mouth and resulting in sustained release of nicotine to the user. The sheet can be in a form that may be placed in the buccal cavity of, on the palate of or sublingually in the user, and have an average dissolution time of 5 to 50 minutes for delivering super bioavailable nicotine to the user.
Owner:PHILIP MORRIS PROD SA
Functional control and age verification of electronic devices through visual communication
An aerosol delivery or electronic nicotine delivery systems (“ENDS”) device may include smoking articles that produce aerosol. The device may operate upon authentication. The authentication may first include an age verification before an authentication allows for operation of the device. The authentication may include a control signal communication to the device. The control signal communication may include an audio signal, such as an authentication tone that is detected by a microphone or pressure sensor on the device. The control signal communication may include a visual, optical, or light signal that is detected by a light sensor or photodiode on the device.
Owner:RAI STRATEGIC HLDG INC
Nicotine containing toiletry waters
InactiveUS20080287507A1Reduce impulseEasy and convenient maskingCosmetic preparationsBiocideLotionToilet
A pharmaceutical liquid formulation for delivering nicotine in any form to a subject by transdermal uptake for treating tobacco dependence and similar conditions, said formulation being a toiletry-water. Toiletry-waters encompass aftershaves, eaux-de-parfum, eaux-de-toilette, eaux-de-cologne, toilet-waters and similar products, all in the form of a lotion, a balm or a gel. Embodiments for certain consideration are aftershave lotions, aftershave balms and aftershave gels.
Owner:MCNEIL AB
Nicotine-oxalate compound crystal and application thereof
InactiveCN111072629AGood sustained release effectFacilitated releaseTobacco treatmentOrganic chemistry methodsCrystallographyOxalate
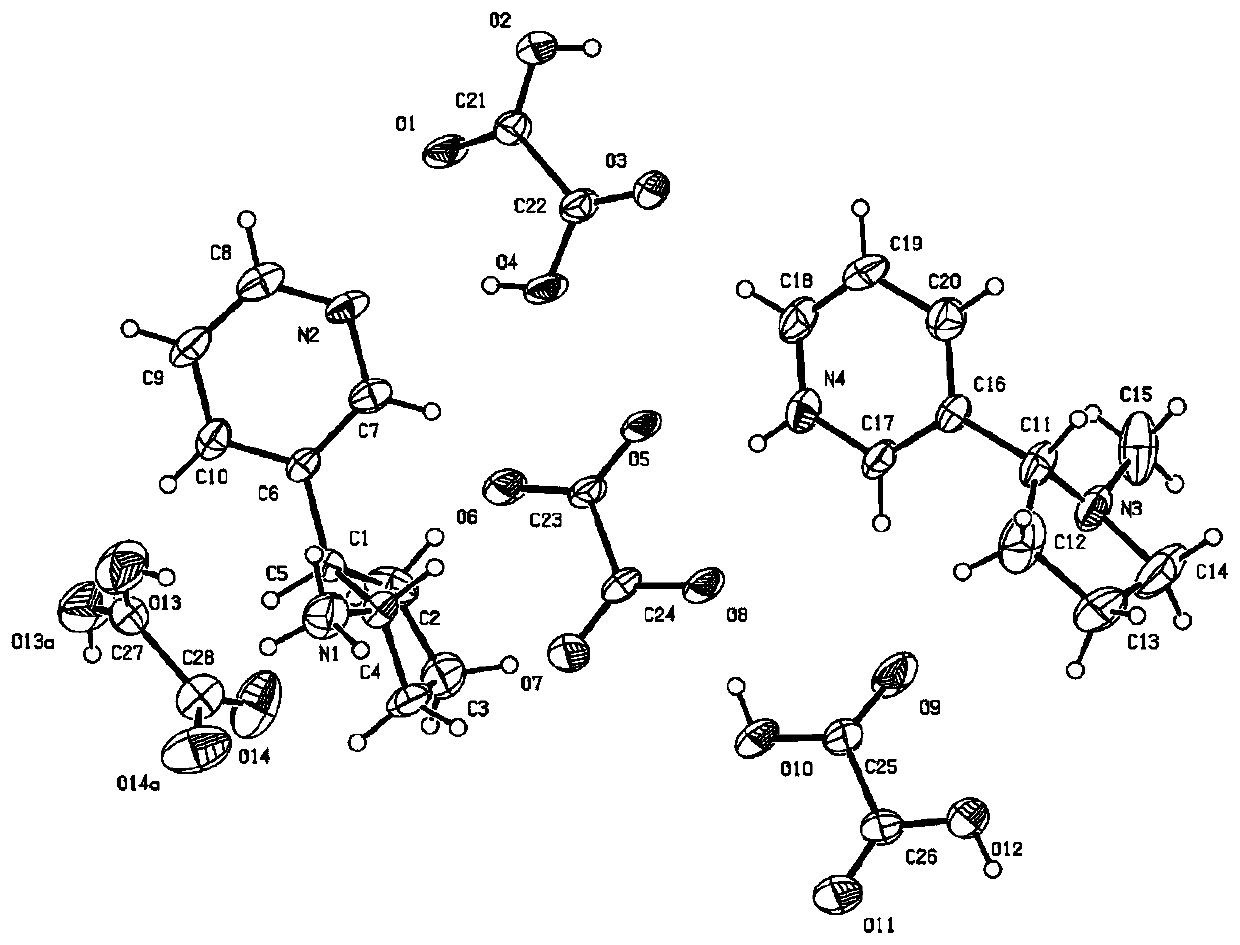
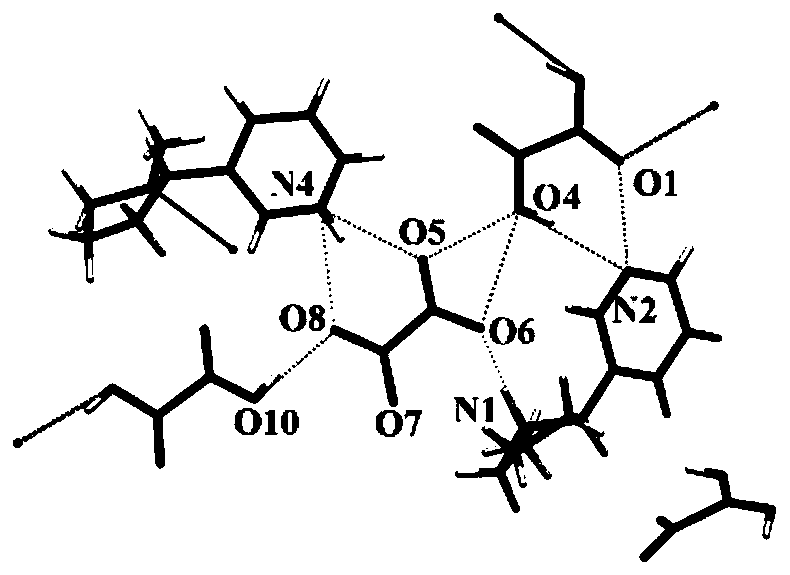
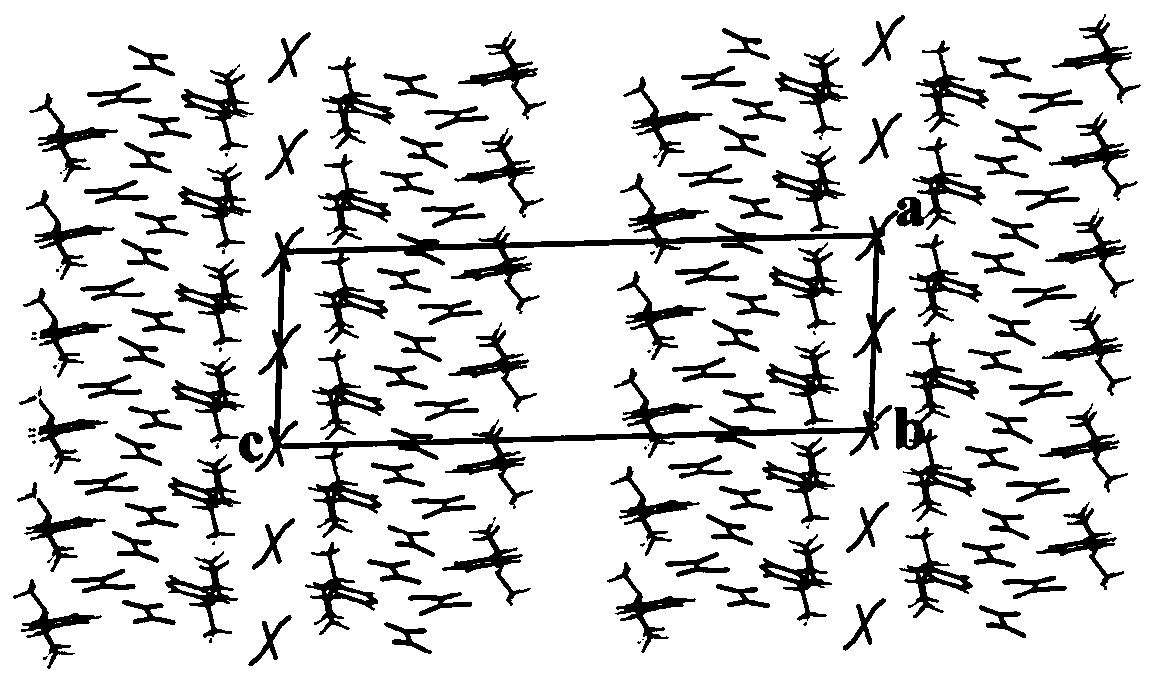
The invention relates to a nicotine-oxalate compound crystal and application thereof, and belongs to the technical field of tobacco chemistry. The molecular formula of the crystal is 2(C10H15N2).C2O4.2.5(C2H2O4). The crystal belongs to a monoclinic system, the space group is C2, and the cell parameters are as follows: a = 10.8406 (15) angstroms, b = 9.8946 (9) angstroms, c = 33.102 (5) angstroms,alpha = 90.00 degrees, beta = 93.481 (13) degrees, gamma = 90.00 degrees and V = 3544.1 (8) angstroms<3>. The nicotine-oxalate compound crystal provided by the invention provides a new introduction form for nicotine of related products such as electronic cigarettes, heat-not-burn cigarettes and the like; when the crystal is used, the taste can be improved, the nicotine delivery process is influenced, and the physiological satisfaction can be continuously provided.
Owner:CHINA TOBACCO YUNNAN IND
Functional control and age verification of electronic devices through speaker communication
An aerosol delivery or electronic nicotine delivery systems (“ENDS”) device may include smoking articles that produce aerosol. The device may operate upon authentication. The authentication may first include an age verification before an authentication allows for operation of the device. The authentication may include a control signal communication to the device. The control signal communication may include an audio signal, such as an authentication tone that is detected by a microphone or pressure sensor on the device. The control signal communication may include a visual, optical, or light signal that is detected by a light sensor or photodiode on the device.
Owner:RAI STRATEGIC HLDG INC
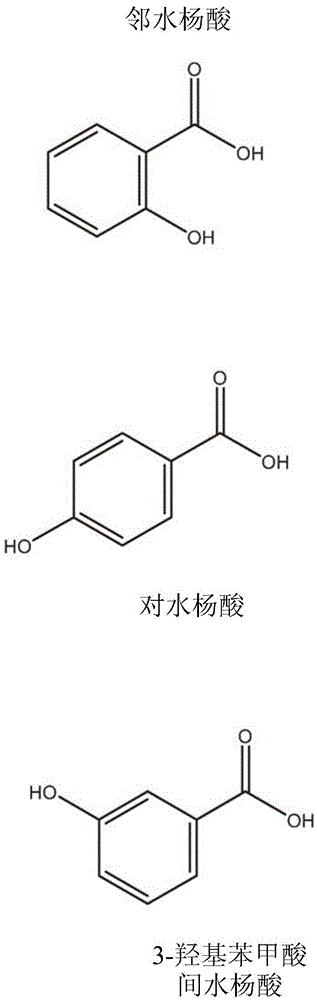
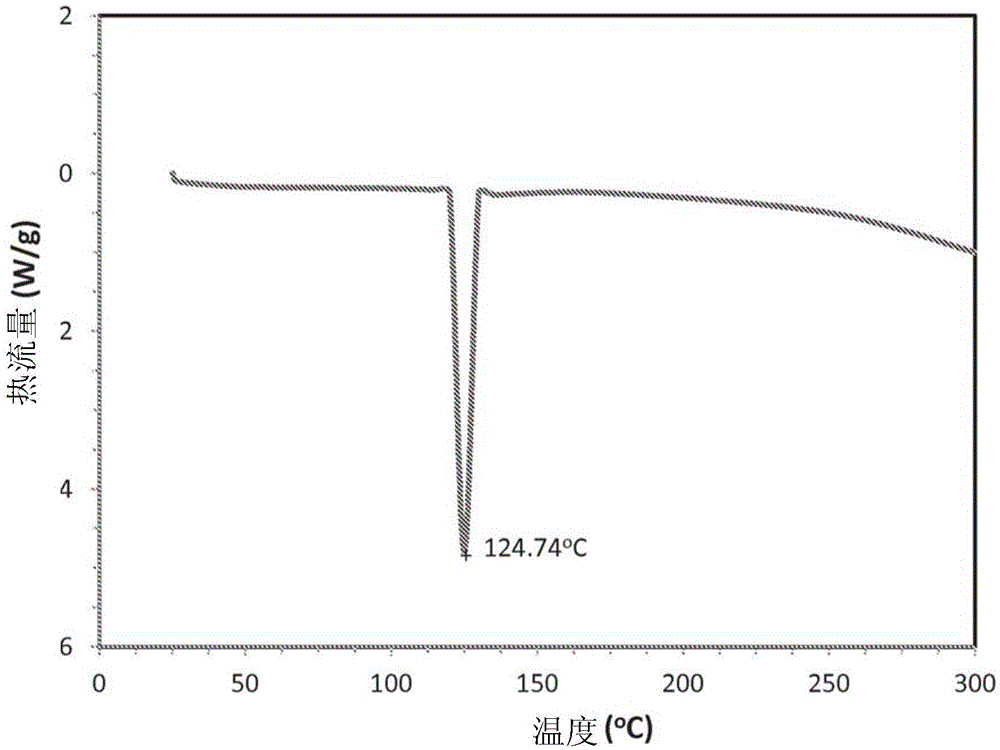
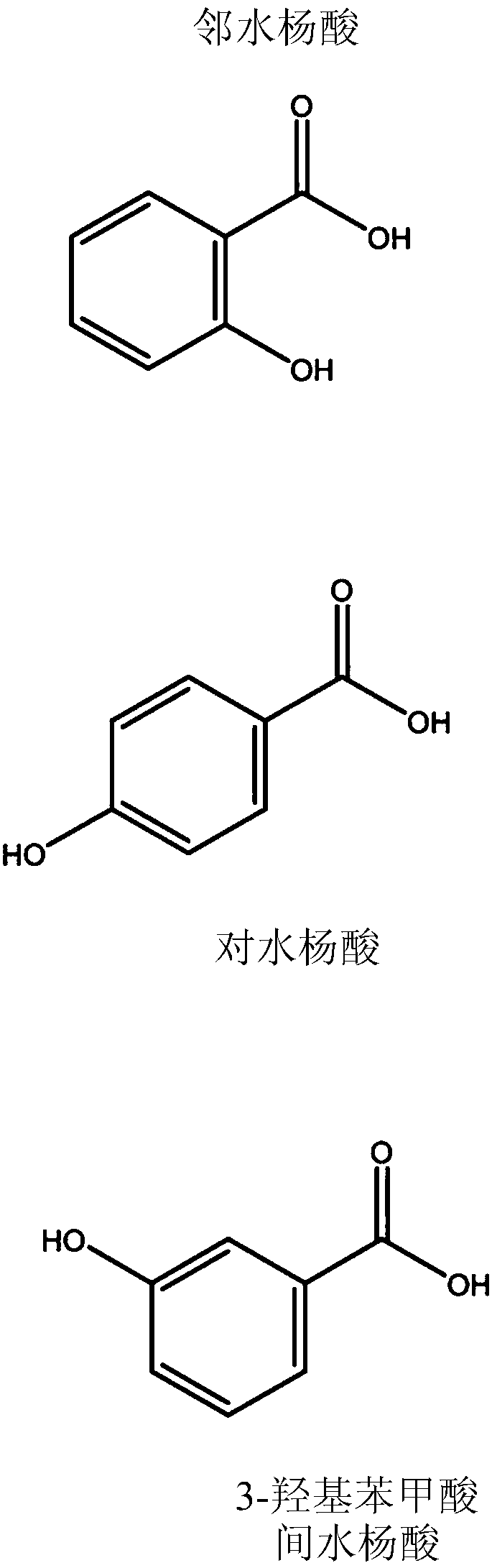
Nicotine salt with m eta-salicylic acid
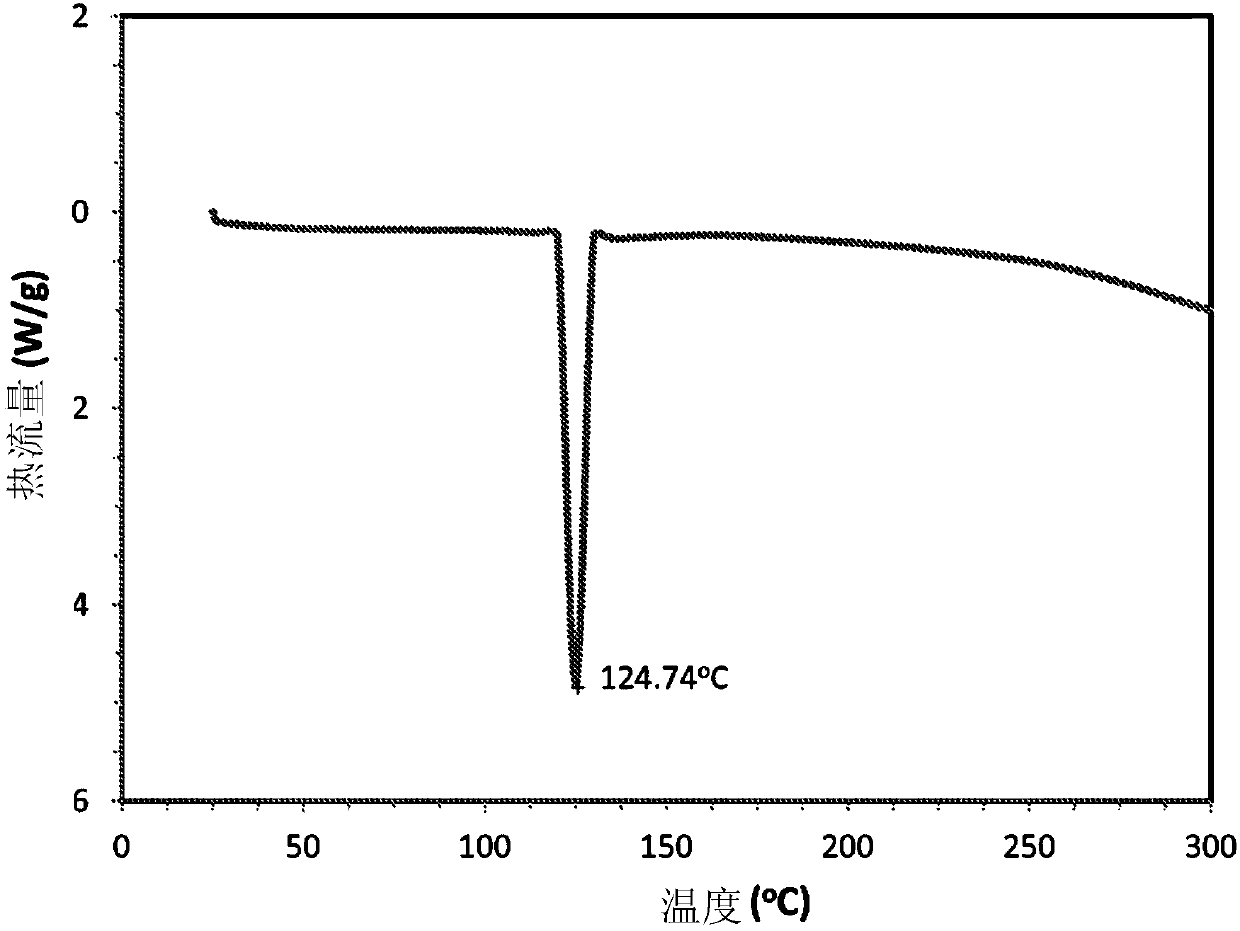
ActiveCN105530916AExothermal chemical reaction heat productionNervous disorderNicotine patchSalicylic acid
The present disclosure relates generally to the field of nicotine delivery. The disclosure teaches a nicotine meta-salicylate. More specifically, the disclosure teaches a condensation nicotine aerosol where nicotine meta-salicylate is vaporized. This disclosure relates to aerosol nicotine delivery devices. The delivery devices can be activated by actuation mechanisms to vaporize thin films comprising a nicotine meta-salicylate. More particularly, this disclosure relates to thin films of nicotine salt with meta salicylic acid for the treatment of nicotine craving and for effecting smoking cessation.
Owner:ALEXZA PHARMA INC
Oral transmucosal nicotine dosage form
InactiveUS20110206621A1Effectively delivered transmucosallyEffectively and rapidly therapeutically effective amountBiocideOrganic active ingredientsNicotine replacementDosage form
The invention described herein relates to an oral transmucosal solid dosage form useful in treating nicotine addiction or as a nicotine substitute or replacement. By virtue of the formulation in combination with nicotine, the invention transmucosally delivers an effective amount of nicotine to the recipient while permitting the accomplishing of such, and manufacture of such, using a relatively small, convenient and orally comfortable dosage form (e.g., tablet) size.
Owner:CEPHALON INC +1
Age verification with registered cartridges for an aerosol delivery device
An electronic nicotine delivery systems (“ENDS”) device may include aerosol delivery devices such as smoking articles that produce aerosol. The device may include a cartridge for the aerosol production. The cartridge is assigned a unique identifier that is approved for a user or that user's device such that the cartridge and / or the device only operate when approved. The unique identifiers help with user identification, tracking, and age verification.
Owner:RAI STRATEGIC HLDG INC
Nicotine delivery product and method for producing it
A nicotine delivery product comprising the reaction product of a nicotine / cation exchange resin complex and an organic polyol; and a method for preparing it comprising (a) mixing an aqueous suspension of a nicotine / cation exchange resin complex with an organic polyol or an aqueous solution thereof, and (b) removing water from the mixture to produce said nicotine delivery product. The nicotine delivery product has a nicotine release rate of at least 80% over a 10 minute period. It is particularly suited for use in smoking substitution devices delivering nicotine such as chewing gum, patches, lozenges, melting tablets and tablets for chewing.
Owner:FERTIN PHARMA AS
Nicotine Salt With M Eta-salicylic Acid
The present relates to a nicotine salt with an m eta-salicylic acid, disclosure relates generally to the field of nicotine delivery. The disclosure teaches a nicotine meta-salicylate. More specifically, the disclosure teaches a condensation nicotine aerosol, wherein nicotine meta-salicylate is vaporized. This disclosure relates to aerosol nicotine delivery devices. The delivery devices can be activated by actuation mechanisms to vaporize thin films comprising a nicotine meta-salicylate. More particularly, this disclosure relates to thin films of nicotine salt with meta salicylic acid for the treatment of nicotine craving and for effecting smoking cessation.
Owner:ALEXZA PHARMA INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com