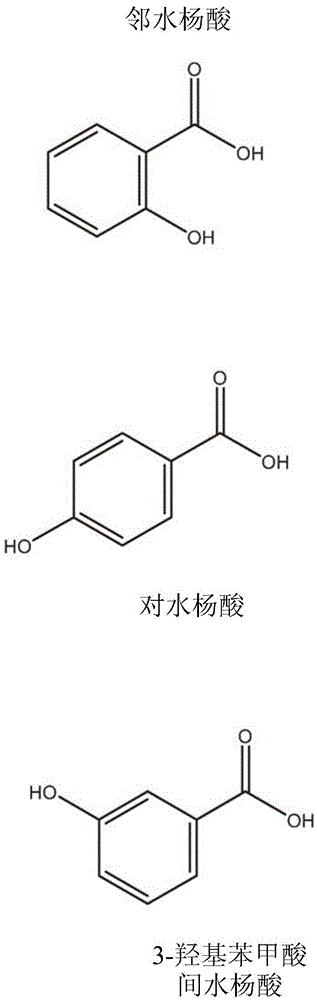
Nicotine salt with m eta-salicylic acid
A technology of salicylic acid and nicotine salt, applied in the fields of nicotine interruption control, condensed nicotine aerosol, aerosol nicotine delivery device, nicotine m-salicylate, and thin film of nicotine m-salicylate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
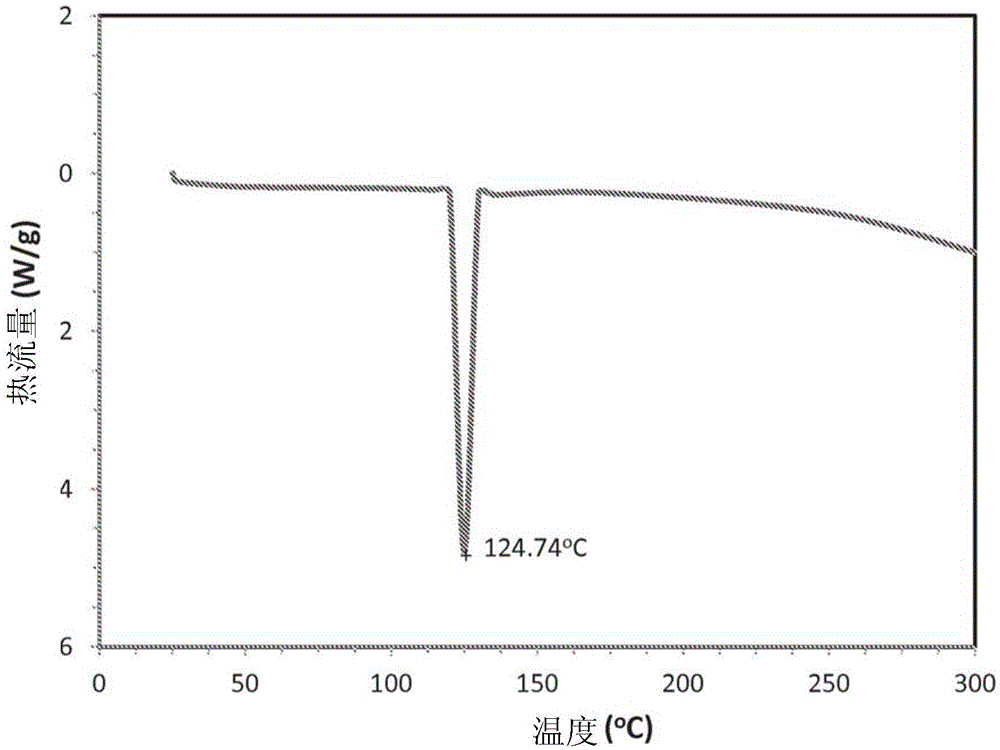
[0211] Synthesis of nicotine m-salicylate: 1.385 g m-salicylic acid (Sigma Aldrich) was dissolved in 25 ml ethanol. 1.62 g of nicotine was added dropwise at room temperature and mixed well for about 30 minutes. The solution was rotevaporated slowly to reduce the volume to about 10ml. Then 5 ml of ethyl acetate was added and stirred for 3-5 minutes. The solution was placed on dry ice for about 1.5 hours, which resulted in a viscous solid mass. After the solvent was evaporated, 20 mL of ethanol was added to dissolve the sticky solid, which was then evaporated. Such recrystallization for purification of crystals is well known in the art. A crystalline solid remained in the flask. The powder was removed from the flask and dried in a vacuum oven at 40°C. 2.6 g were recovered (about 87% yield). The melting point of the solid is 125°C.
Embodiment 2
[0213] Synthesis of nicotine m-salicylate: liquid nicotine (AlfaAesar, batch #10150504, 99% purity) and m-salicylic acid (3-hydroxybenzoic acid, SigmaAldrich, batch #STBB7747, 99% purity) were prepared in 1 A :1 nicotine:acid ratio is used to synthesize nicotine m-salicylate. The following synthetic route gives a typical yield of 60-70% and a purity of 99.7%.
[0214] Sample synthesis:
[0215] 1. Dissolve 0.03 molar meta-salicylic acid (-4.16 g) in 65 ml ethanol (200 proof) and mix well for about 20 minutes.
[0216] 2. Add 0.03 molar liquid nicotine (-4.86 g) dropwise to the above solution and mix on a stir plate for 30-40 minutes with periodic shaking. Seed crystals of nicotine m-salicylate were then added and the solution was stirred for an additional 40 minutes and then placed on dry ice for about 40 minutes.
[0217] 3. Filter and wash nicotine m-salicylate with 100% acetone and air dry for ~20 minutes; to homogenize the salt, use a mortar and pestle, then dry the sal...
Embodiment 3
[0220] API evaluation:
[0221]Nicotine m-salicylate raw material (API) was characterized using various analytical methods. The result proves that the synthesized nicotine m-salicylate has high purity.
[0222] A typical calorimetric scan of nicotine m-salicylate powder is shown in figure 2 middle. The melting point is 125°C. The powders were subjected to thermogravimetric analysis in both scanning and isothermal modes. Figure 7 Displays scan data from room temperature to 500°C. It is noteworthy that the flat baseline was approximately (actually, slightly below) 0%, indicating that little or no residue was left. Weighing the pans before and after external equilibration left only ~0.2%. This result suggests minimal carbonization of the acid after exposure to high temperature. Isothermal data were also obtained for API powder (-10 mg per run) at 40°C, 50°C, and 60°C over a period of at least 3 days. In all cases, the mass change was essentially a linear decrease with t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com