Iontophoretic transdermal delivery of nicotine salts
A nicotine salt and nicotine technology, applied in electrotherapy, medical preparations containing active ingredients, treatment, etc., can solve problems such as undesired attention, difficult to conceal places, uncomfortable patches, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1: Transdermal Delivery of Nicotine Base by Passive Diffusion and Iontophoresis
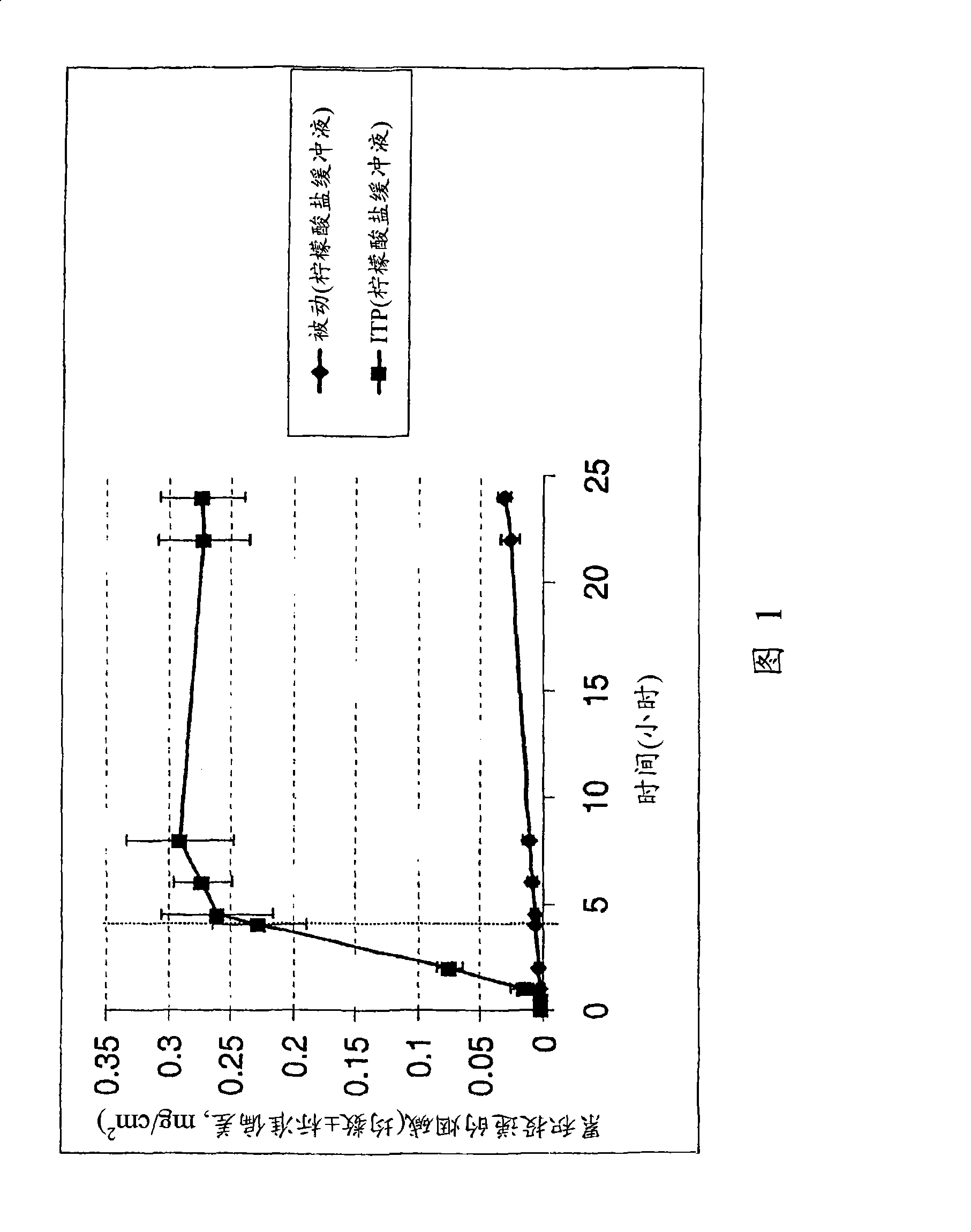
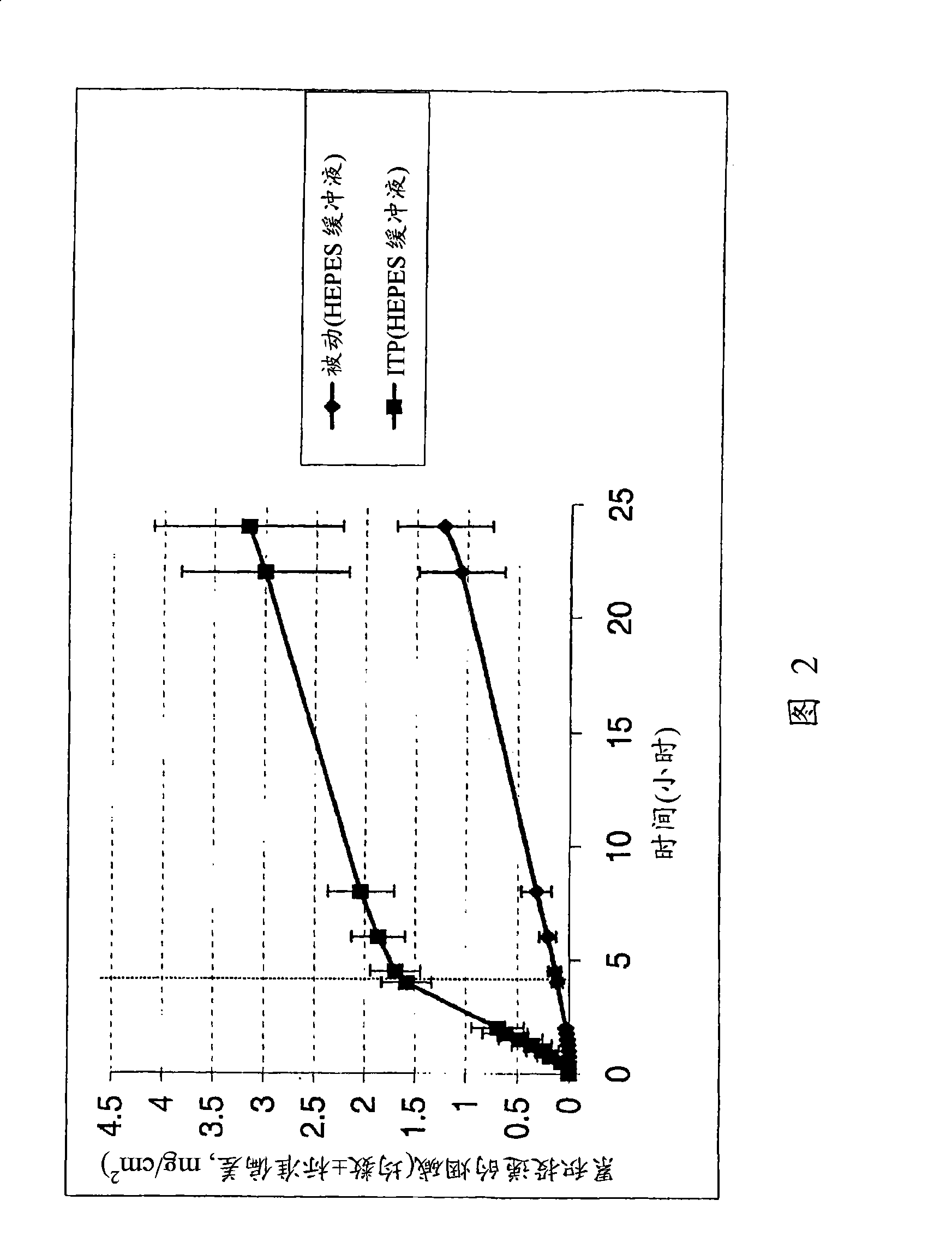
[0022] Studying the Permeation of Nicotine Base in Two Different Setup Conditions
[0023] i) Nicotine in 50 mM HEPES ([4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) buffer (pH 5.5) containing 50 mM NaCl.
[0024] ii) Nicotine in 500 mM citrate buffer (pH 8) containing 50 mM NaCl.
[0025] Nicotine is a binary base with pKa values of 3.4 and 8.2. At pH greater than 9, it is a free base. At pH 4.8-7.5, it is in the form of free base and single cation. Nicotine is more passively transported at pH 8, where the drug is present primarily in the nonionic form, compared to the monocationic form at pH 5.5. In both cases evaluated above, iontophoresis enhanced nicotine penetration compared to passive diffusion nicotine delivery (Figures 1 and 2).
Embodiment 2
[0026] Example 2: Transdermal Delivery of Nicotine Salts by Passive Diffusion and Iontophoresis in 500 mM Citrate Donor Buffer
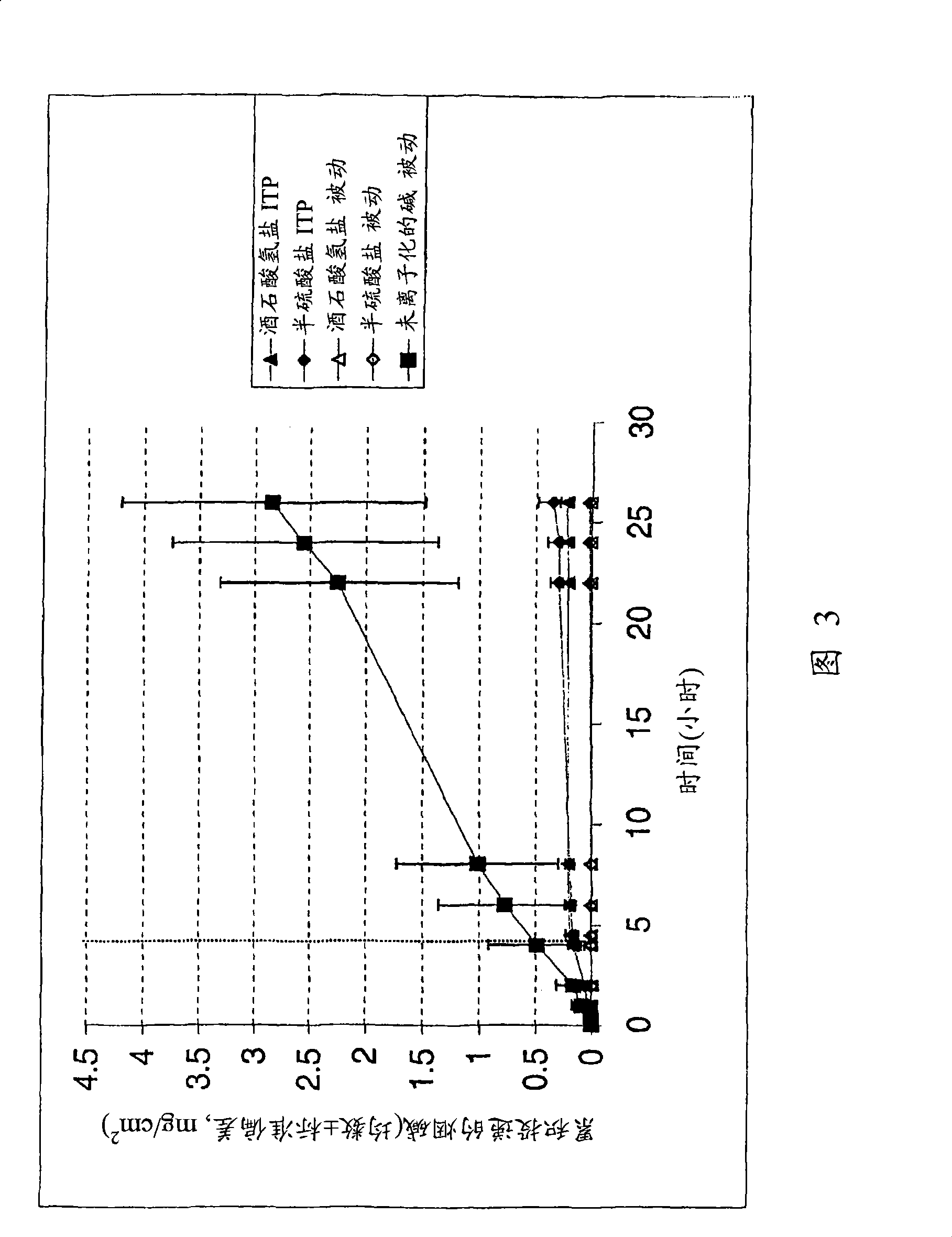
[0027] Study of passive diffusion and iontophoresis of nicotine salts (equivalent to 1% nicotine), especially nicotine bitartrate and nicotine hemisulfate, in 500 mM citrate buffer containing 50 mM NaCl . The iontophoresis current was stopped after 4 hours. The iontophoretic flux of nicotine was similar in both salt forms, which was less than that of passive delivery of nicotine base at pH 8 (Figure 3). The pH of the donor solution was checked after the experiment, and it was found that the pH of the donor solution did not change.
Embodiment 3
[0028] Example 3: Comparison of Iontophoretic Delivery of Nicotine Bitartrate in 50 mM HEPES Donor Buffer vs. 500 mM Citrate Donor Buffer
[0029] Iontophoresis of nicotine bitartrate was studied in 50 mM HEPES buffer (pH 5.5). Nicotine bitartrate lowered the pH of the HEPES buffer to about 3.5, which was then adjusted to about 5.5 with NaOH.
[0030] As can be seen from the comparative graph (Figure 3), the flux of nicotine from the 50 mM HEPES buffer is higher than that from the 500 mM citrate buffer. It appears that the higher buffer strength citrate buffer contributed more buffer ions, which competed with drug ions for passage through the skin, thus reducing nicotine penetration. This has some indication in the conductivity test. 500 mM citrate buffer has a conductivity of 66400 μmhos / cm compared to 16300 μmhos / cm for 50 mM HEPES buffer. A final pH check of the experiment in a 50 mM HEPES solution showed no significant change in pH.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com