Most conventional medicine delivery systems have limitations which make them less than ideal.
One limitation relates to the speed of delivery.
The delay in pharmacological effect is especially problematic in situations where the patient takes the medication in response to a stimulus.
In those patients, dangerous complications could arise if the medication is delivered ineffectually or at too fast of a rate.
Another limitation relates to variations in concentration of medicine achieved in the user's bloodstream.
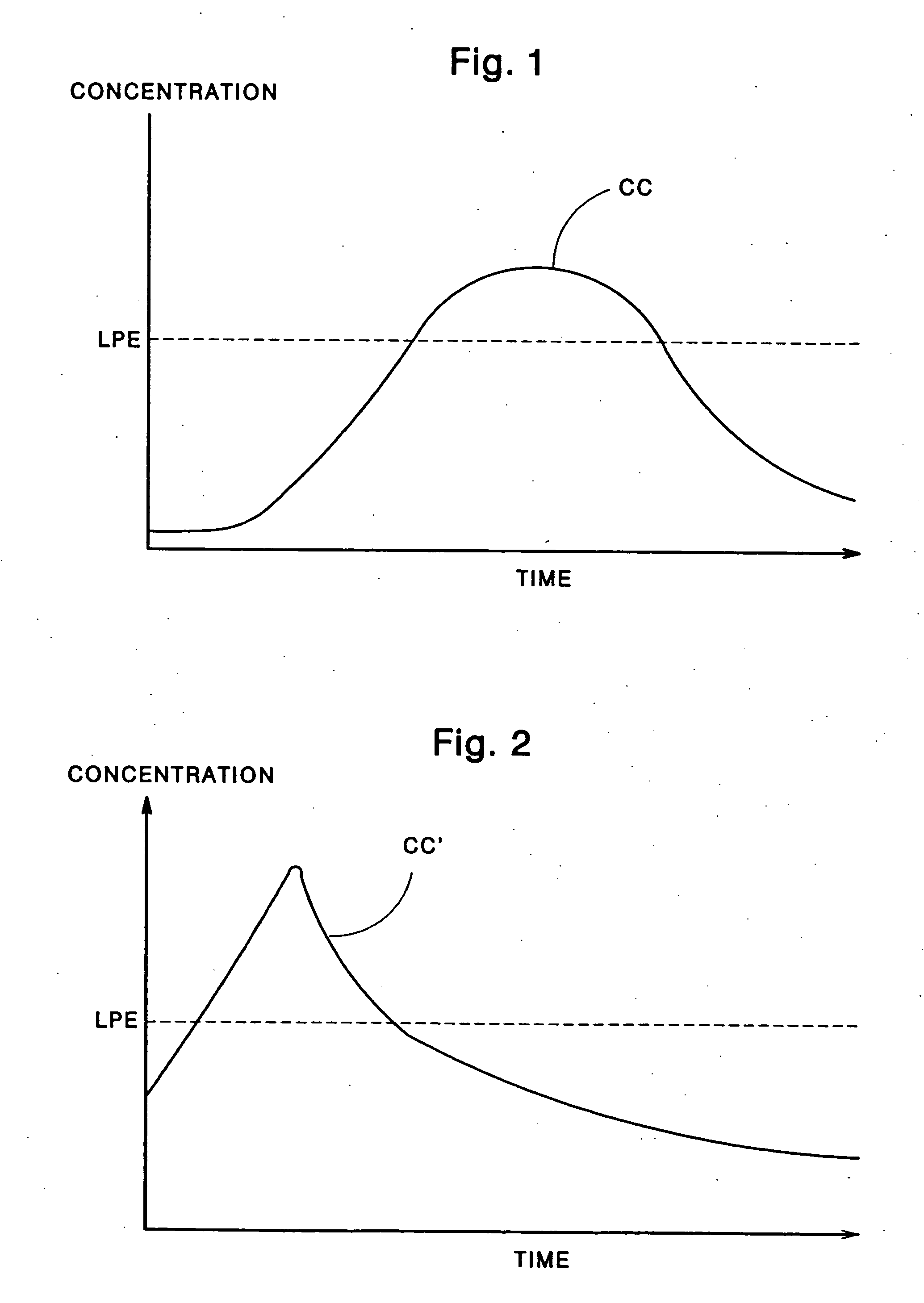
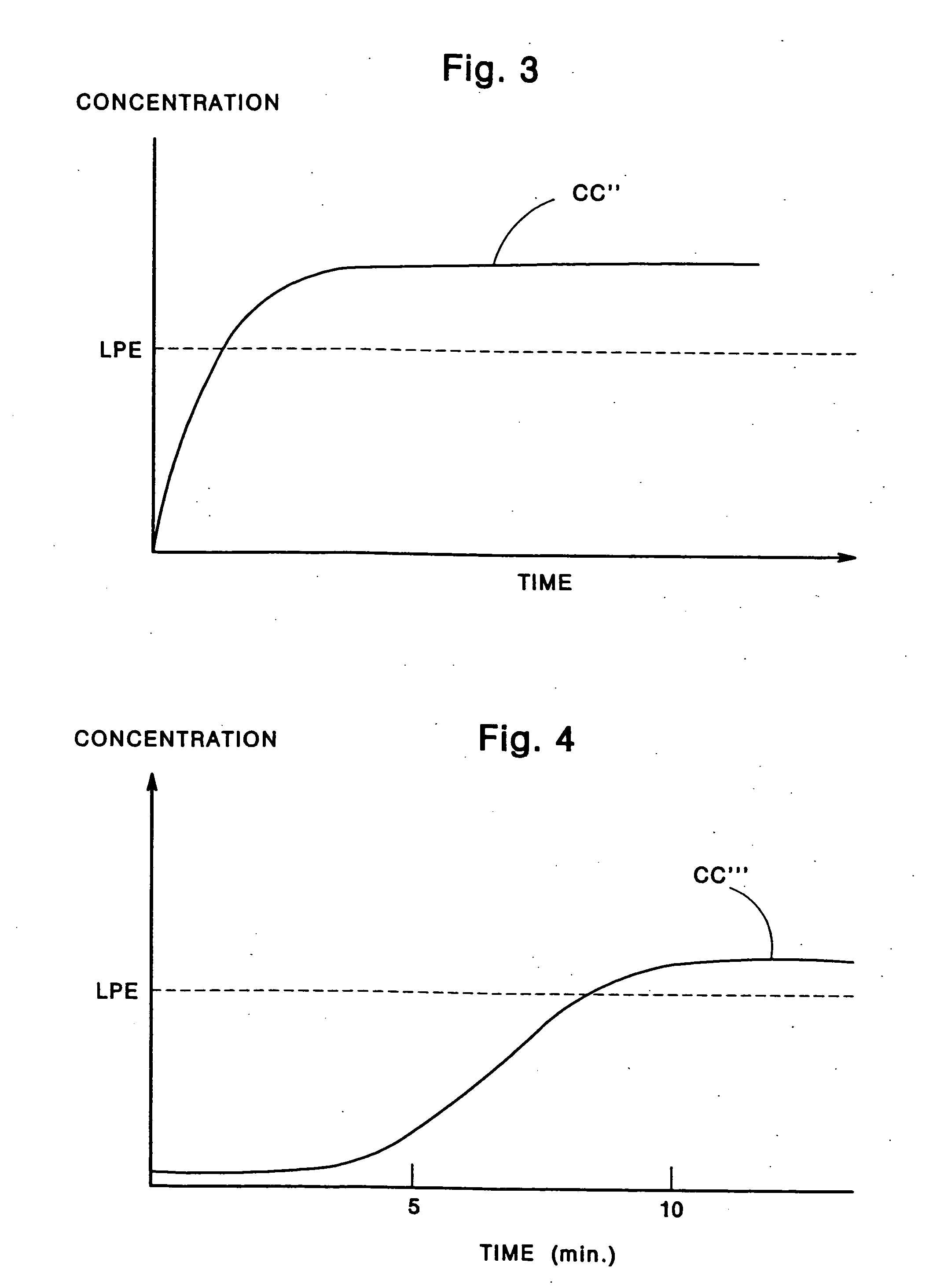
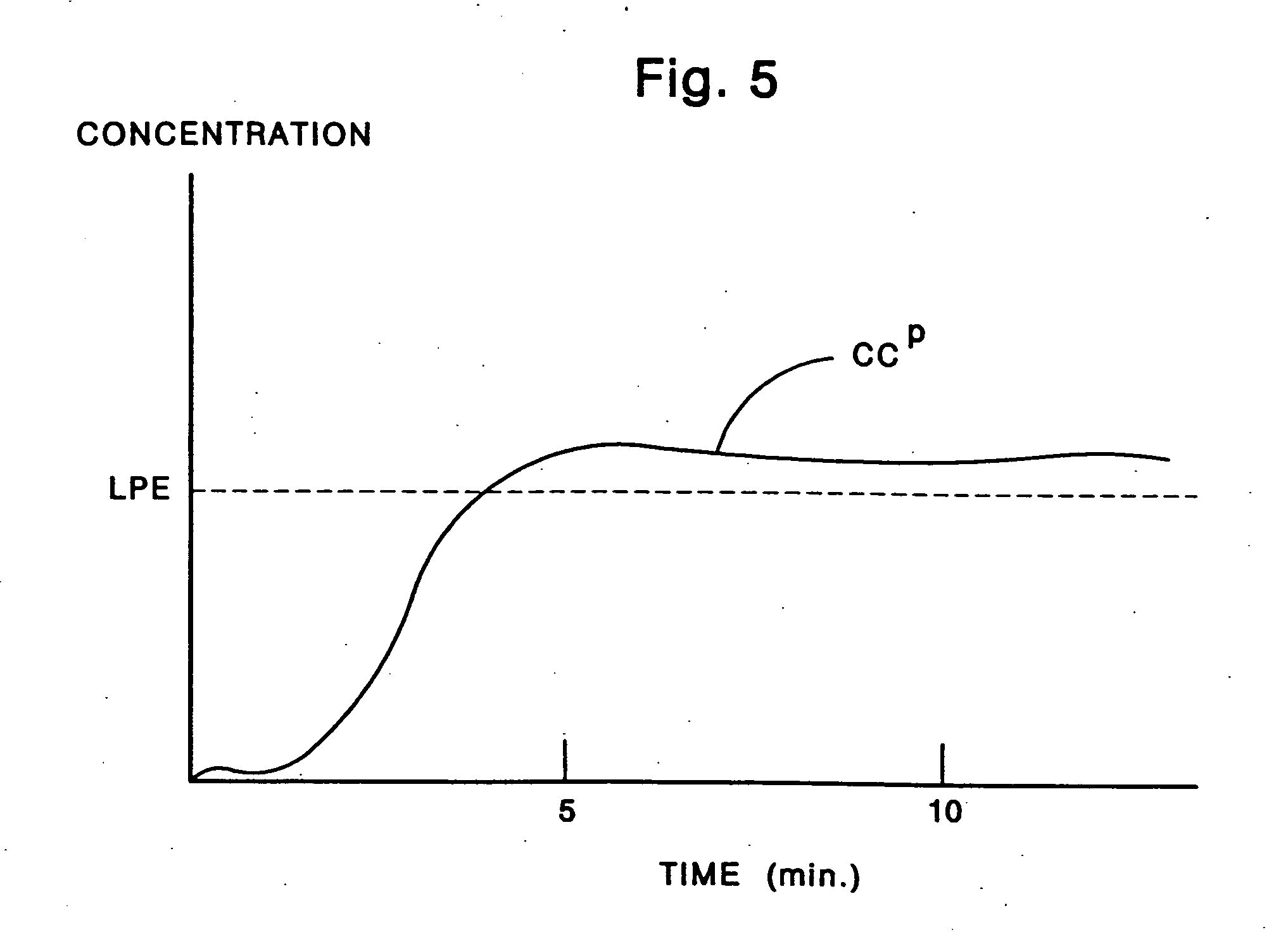
Few, if any, medicine delivery systems can provide substantially constant concentrations of medicine in the bloodstream.
Thus, when a medicine delivery system provides such low concentration, it is doing little, if anything, to alleviate the patient.
Similarly, concentrations of medicine above the LPE typically are unnecessary, and can produce side-effects or reactions to the medication.
Conventional oral delivery systems fail to provide the foregoing combination of doses and the desirable results associated therewith.
The resulting overdosage tends to produce toxic side-effects or reactions to the medication.
The length of this delay may vary and is affected by the speed of ingestion.
This represents a potentially unacceptable delay in the desired pharmacological effect.
This delay is particularly unacceptable where the medicine is used to reduce cravings.
In those situations, the delay may be long enough that the patient succumbs to the craving.
Although the period of time between pills can be decreased in an attempt to reduce the magnitude of the overdosages, this becomes inconvenient to the patient and greatly increases the likelihood that the patient will not comply with the dosage requirements and therefore will not receive the full benefit of the desired pharmacological effect.
An additional problem with conventional oral medicine delivery systems which require stomach absorption of the medicine is the highly volatile enzymatic environment of the gastro-intestinal system.
This environment can alter the medicine and reduce or eliminate its effectiveness.
Such injection techniques, however, fail to provide constant concentrations of the medicine in the blood over prolonged periods of time.
The hypodermic injection technique therefore exposes the patient to an over-dosage.
This over-dosage, in turn, increases the likelihood of side-effects and adverse reactions.
Another disadvantage associated with the conventional hypodermic injection technique is that it fails to provide a prolonged period of time during which the concentration remains at or near the LPE.
Yet another disadvantage associated with conventional hypodermic injection techniques is the pain associated with such injection techniques.
Some patients are extremely disturbed by the notion of hypodermic injection.
This limits the number of patients which will use the injection technique.
When all three requirements are present, the injection technique is extremely inconvenient to the patient.
This can result in delay because medical personal usually cannot respond immediately.
The requirement of medical personnel also makes the intravenous drip technique very inconvenient for the patient.
Another disadvantage associated with the intravenous drip technique is the need to insert a catheter subcutaneously.
The initial insertion may be painful and uncomfortable, making this technique difficult on patients.
In addition, the patient's mobility may be significantly hampered during the intravenous delivery of the medicine.
The intravenous drip technique is especially difficult to implement on an effective basis when the medicine is a craving reduction medicine.
In those situations, it is impractical to have the patient meet with medical personnel to have a catheter inserted every time the patient experiences a craving.
Although such techniques may provide rapid and effective pharmacological effects, it is difficult to regulate the concentration of medicine in the blood over a prolonged period of time when such techniques are used.
A disadvantage associated with conventional gum chewing techniques, however, is the delay between the time when chewing begins and onset of the desired pharmacological effect.
The delay typically is caused by the time it takes for sufficient medicine to be released from the gum and for that medicine to be absorbed into the bloodstream.
In the case of gums which are used to counteract cravings for nicotine-containing products, a substantial portion of the nicotine from such gums may be swallowed because of poor absorption in the mouth.
Since nicotine, when swallowed, can cause adverse gastrointestinal symptoms, such as hiccupping and nausea, the conventional chewing gum technique can produce undesirable side-effects.
If the gum fails to provide a desired level of craving relief, attempts to obtain additional nicotine from the gum may cause increased feelings of nausea because of the frequent failure of users to absorb (rather than swallow) the nicotine.
Thus, the effectiveness of conventional nicotine delivery gums may be difficult to adjust upwardly without experiencing an increased potential for nausea.
When the dose is increased, for example by using the 4 milligram version of Nicorette™ (which delivers about 2 milligrams of nicotine) or by administering multiple units of Nicorette™ (up to 4 units of the 4 milligram version of Nicorette™), the reliability of the craving reduction increases, but the probability of undesirable consequences, such as dizziness and nausea, also increases.
This initial delay, however, may be excessively long for someone who is trying to quit smoking.
While the initial burst of nicotine is relatively rapid when compared to that provided by most other commercially available transdermal nicotine patches, it is still slow (about 30 minutes to achieve a significant increase in nicotine blood level) when compared to what can be achieved by delivery through the mouth.
The relationship of the pH to the absorption rate of nicotine also was documented by O. Ferno in 1977, who concluded that unbuffered formulations were ineffective at nicotine delivery or relieving withdrawal symptoms.
 Login to View More
Login to View More