Patents
Literature
84 results about "Buccal mucosa" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
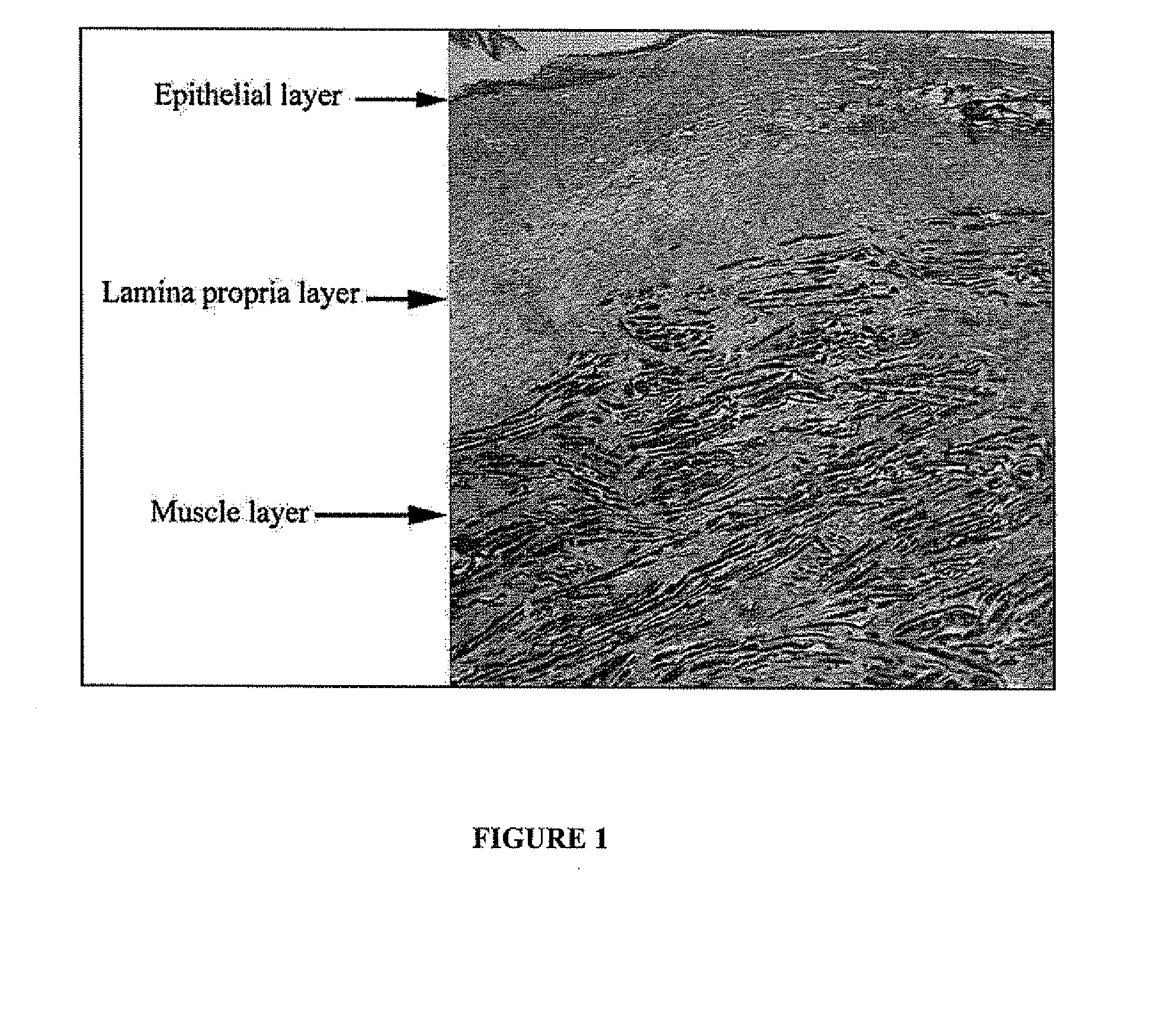
The buccal mucosa is a part of the mucous membrane covering the mouth area. Drinking plenty of water is important to keeping the buccal mucosa lubricated. The parotid gland, which produces saliva for the mouth, flows into an area of the buccal mucosa near the second upper molar.
Nicotine-containing pharmaceutical compositions giving a rapid transmucosal absorption
Formulations of nicotine for use in nicotine replacement therapy. The formulations are intended for application in the oral cavity where upon the uptake of nicotine mainly takes place through the buccal mucosa. The formulations essentially comprise apolar, polar and surface-active components. The formulations may be administered in combination with other nicotine formulations.
Owner:MCNEIL AB
Combination tablet with chewable outer layer
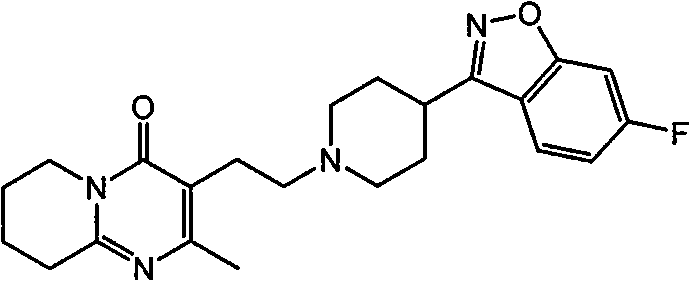
ActiveUS8404275B2Improve the level ofAbsorbed more rapidlyBiocideAnimal repellantsDosing regimenSide effect
A pharmaceutical composition in the form of a combination tablet is described. The tablet has a rapidly absorbed component that enters the circulation by traversing the buccal mucosa, oral mucosa and combinations thereof, and a more slowly absorbed component that is swallowed. The therapeutic agent in the swallowed portion is absorbed across the gastric mucosa. The combination tablet may be modified, by varying the specific combinations of excipients, fillers, and the like to effect distinct release rates. In addition, the rapid and slow components may have identical or different therapeutic agents depending on the application to a specific medical condition. One embodiment of the combination tablet includes a prostaglandin inhibitor in the rapidly absorbed component in order to mitigate the side effects of immediate release niacin that is in the slow absorbing component. Such combination compositions will increase patient compliance with various dosing regimens due to the resultant decrease in the number of tablets that a patient would need to take on a daily basis.
Owner:VITALS
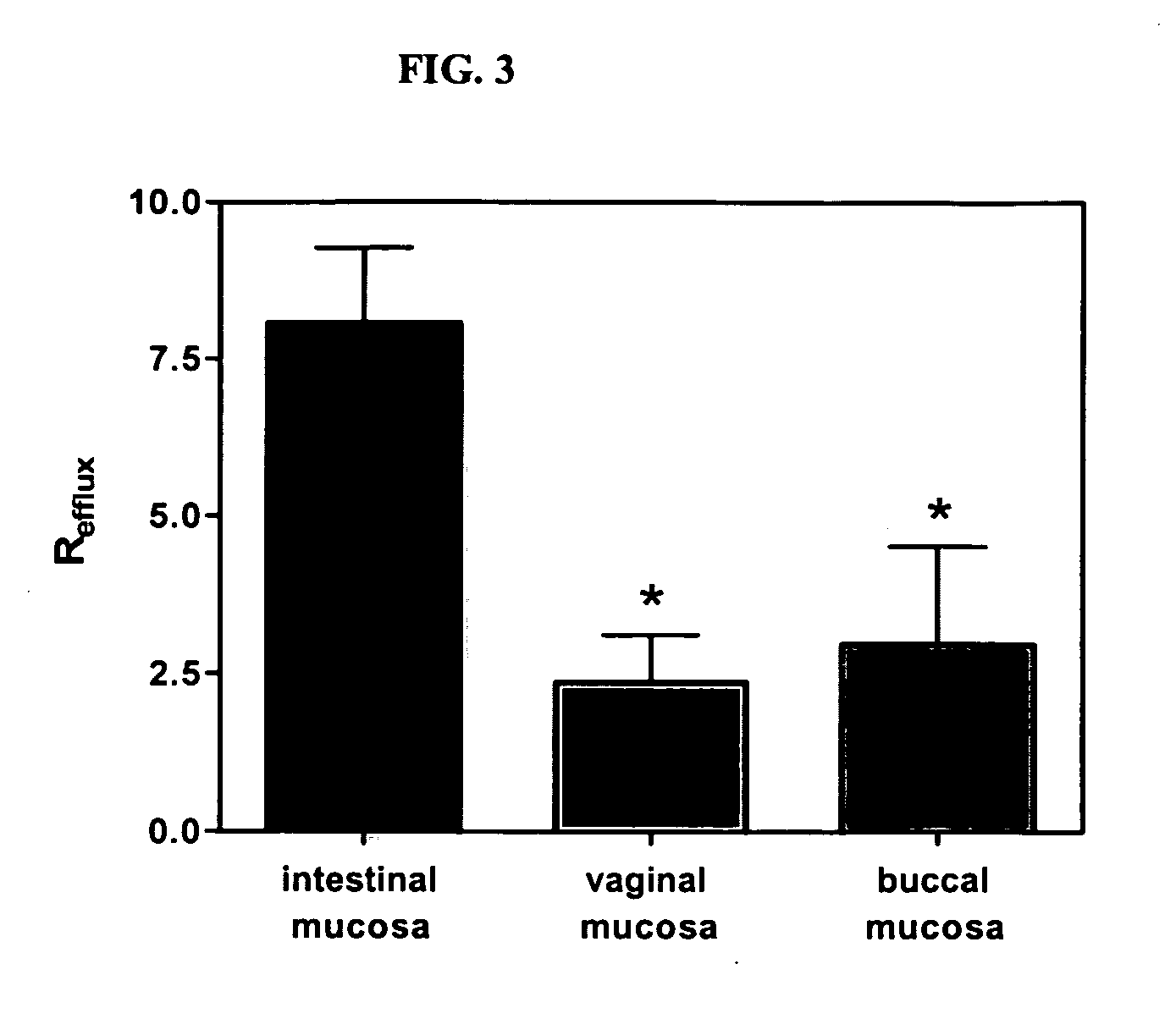
Method for augmentation of intraepithelial and systemic exposure of therapeutic agents having substrate activity for cytochrome P450 enzymes and membrane efflux systems following vaginal and oral cavity administration
InactiveUS20070036834A1Improve bioavailabilityImprove drug solubilityBiocidePowder deliveryWhole bodyMedicine
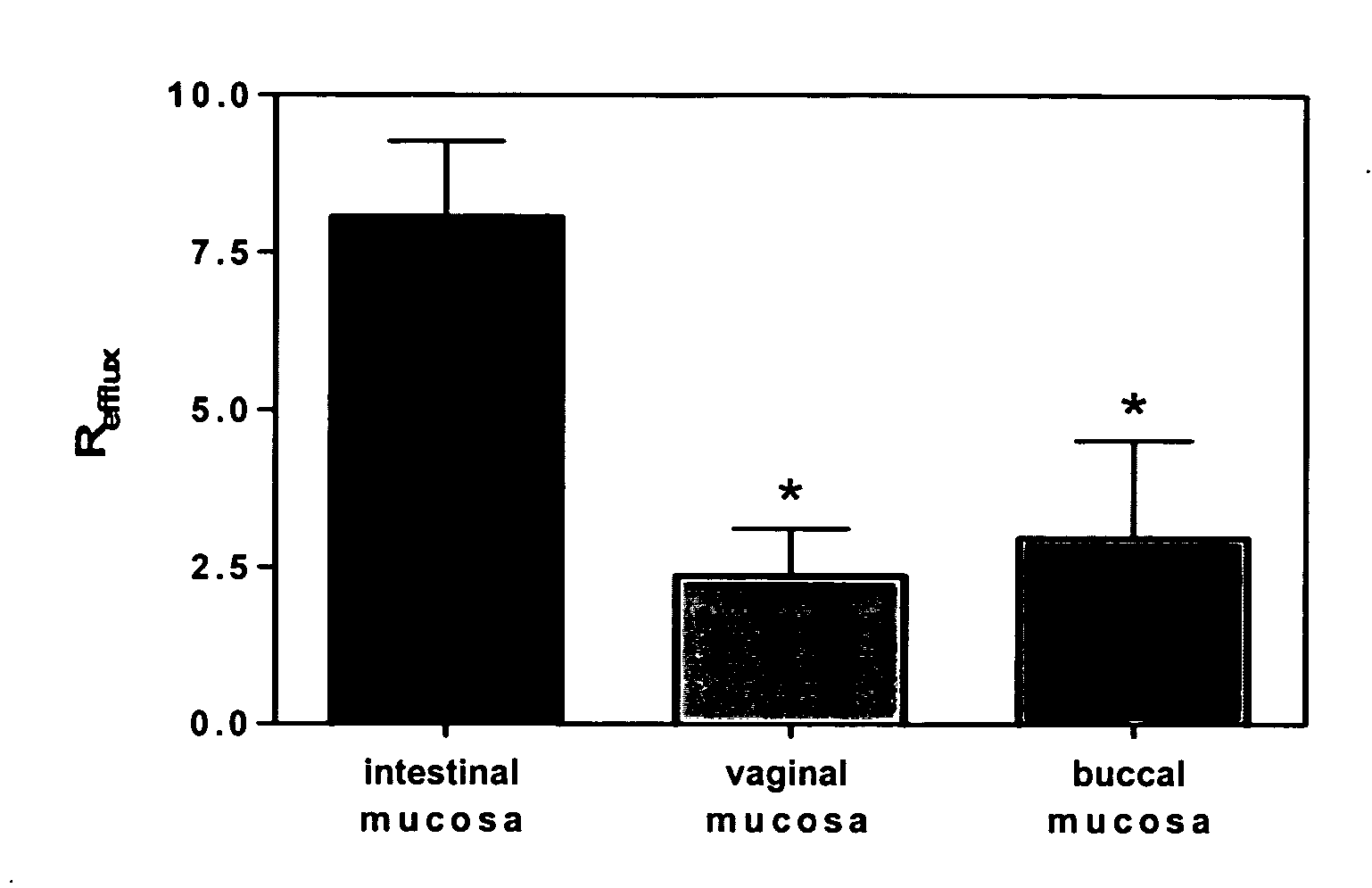
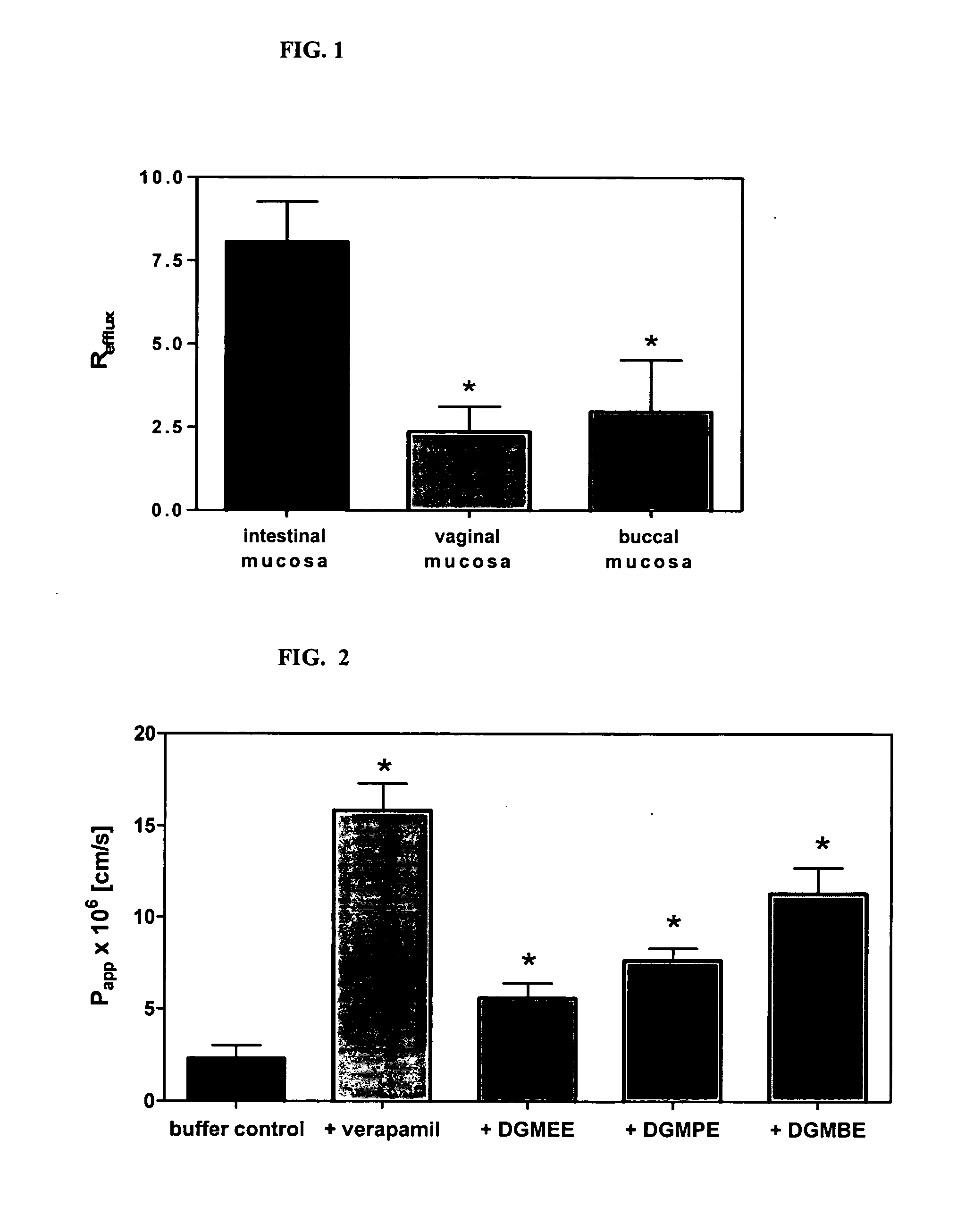
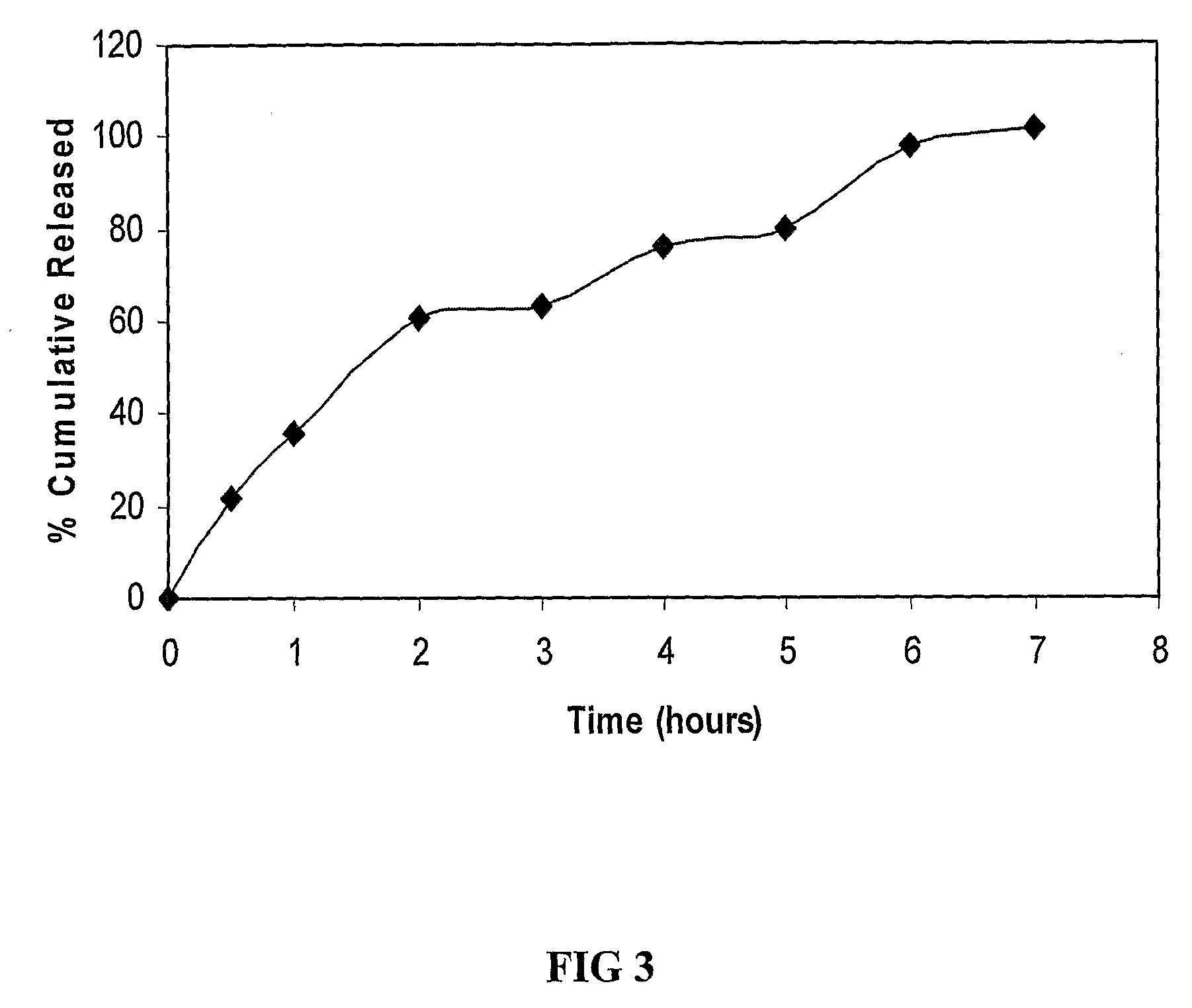
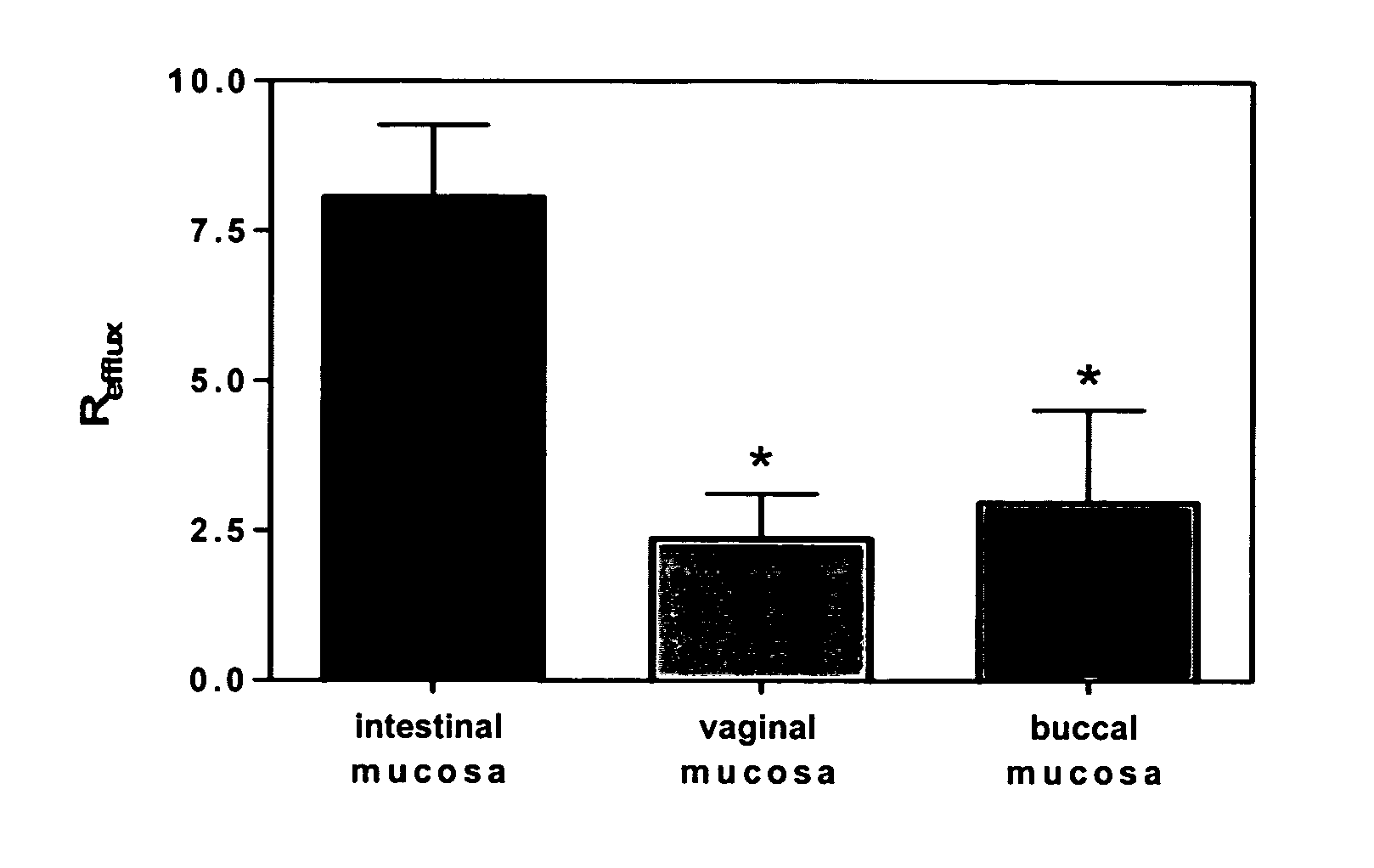
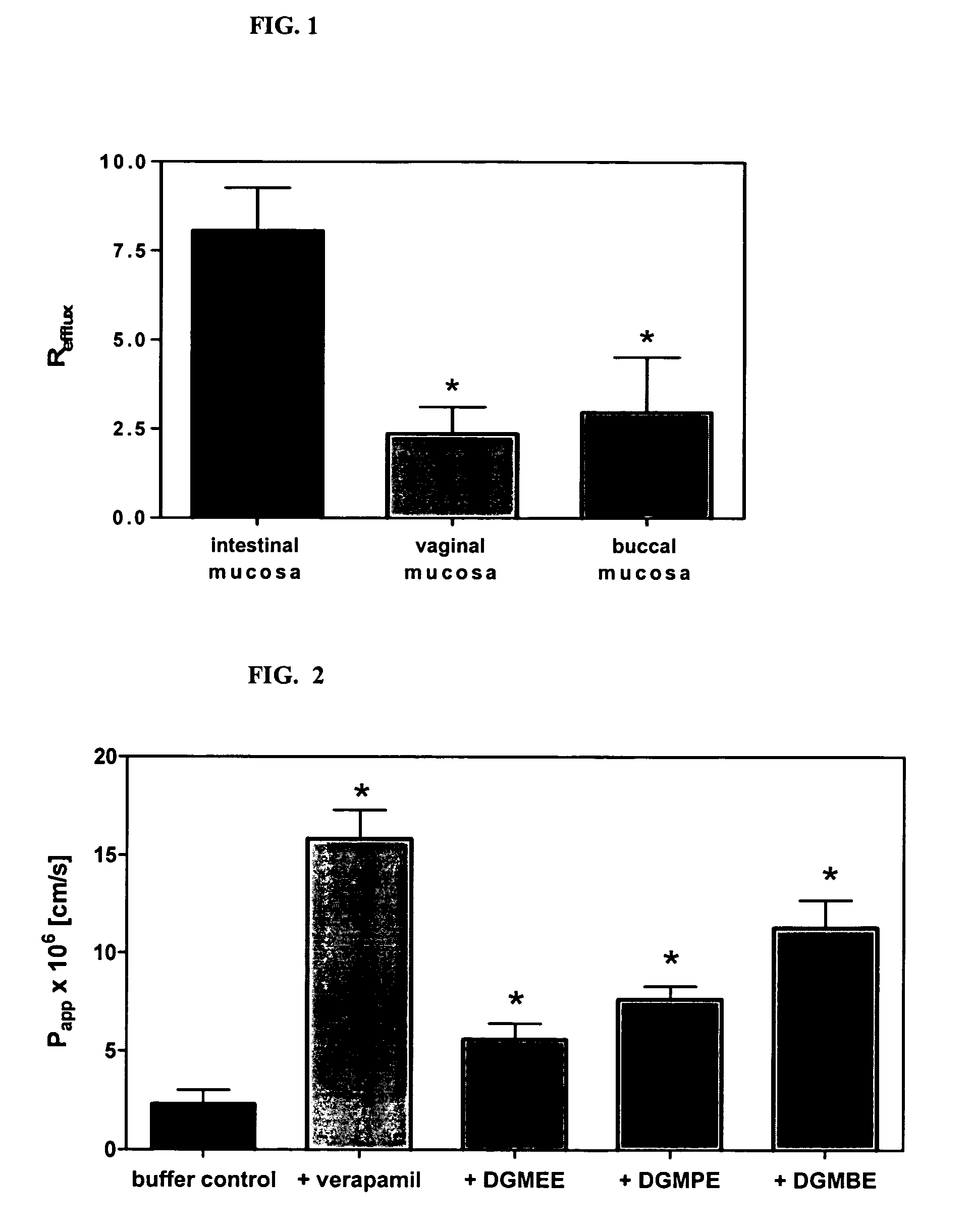
A vaginal or buccal delivery of therapeutic agents having a substrate affinity for metabolic cytochrome P-450 enzymes and membrane efflux transporter systems. A method for augmentation of systemic exposure to the therapeutic agents having a substrate affinity for cytochrome P-450 enzymes and membrane efflux transporter systems, by delivering said agents to the systemic circulation through vaginal or buccal mucosa.
Owner:FEMINA PHARMA +1
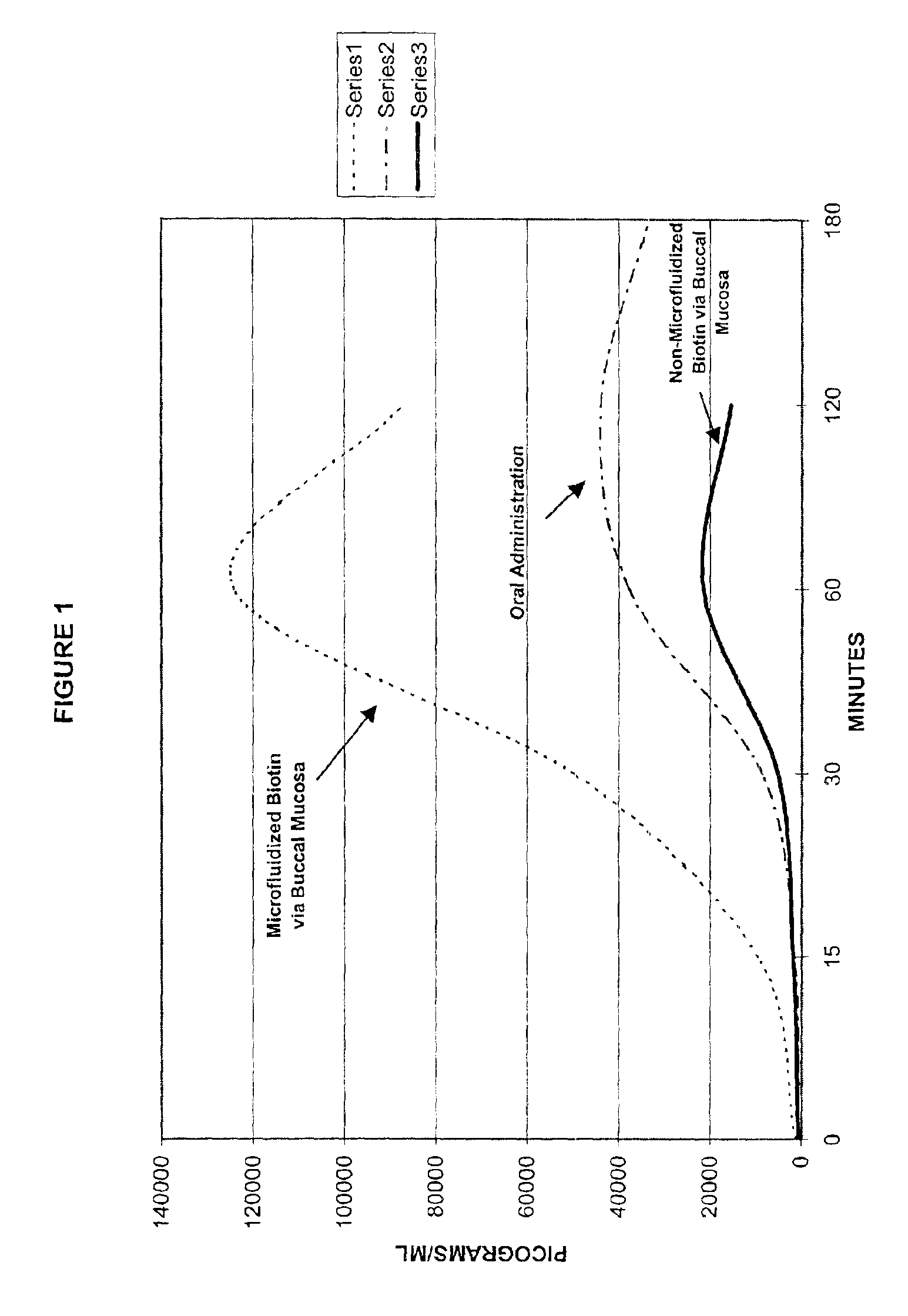
Method for the delivery of a biologically active agent
InactiveUS6861066B2Reduce deliveryIncrease concentrationPowder deliveryGogglesControlled releaseActive agent
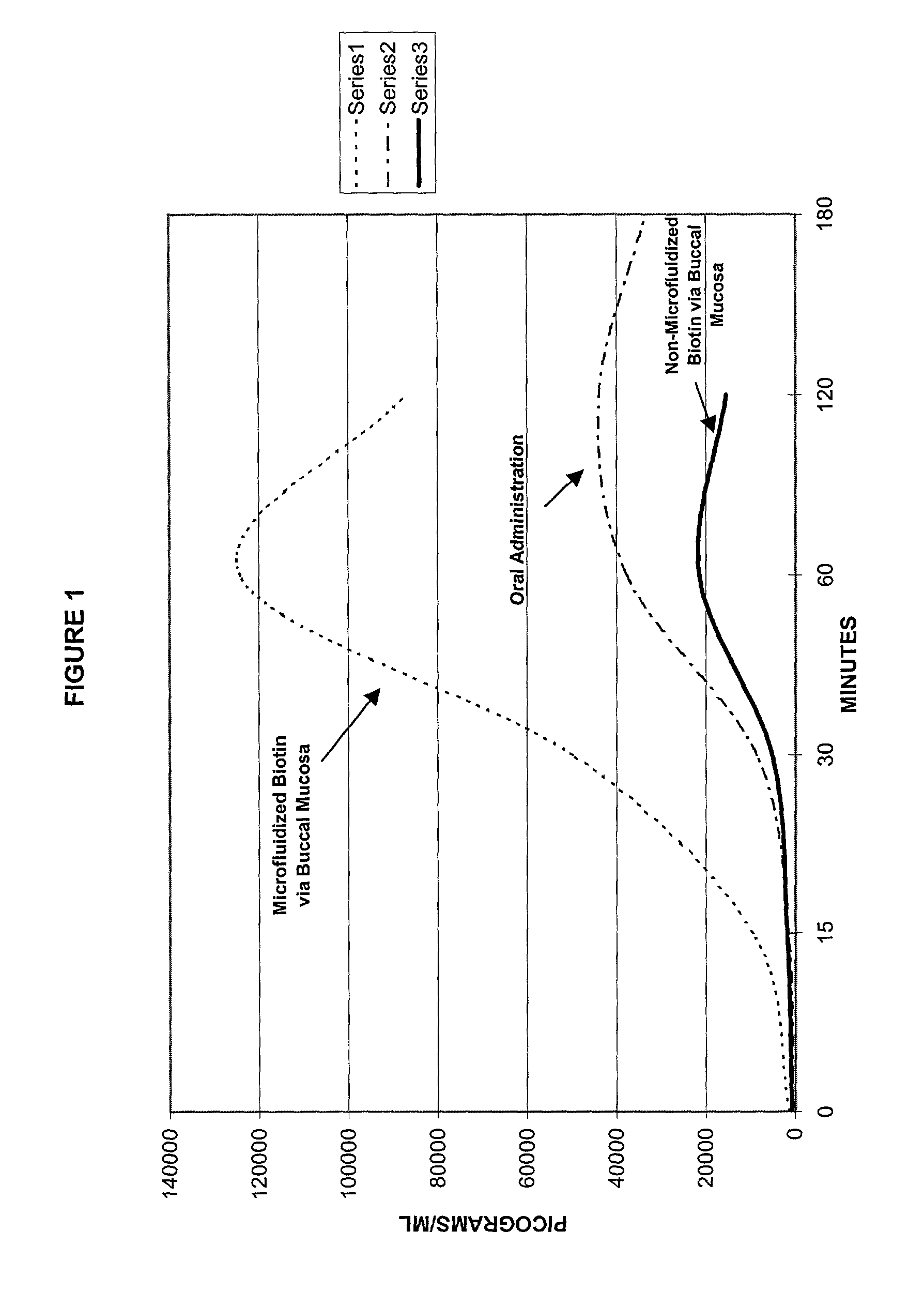
A method of manufacturing a stable nanosuspension for delivery of a biologically active agent into the bloodstream of a subject is disclosed. A microfluidizable mixture is initially formed and processed via a microfluidization process to form the stable nanosuspension, which may be administered via the buccal mucosa or other suitable routes of administration. This product demonstrates increased bioavailability, enhanced period of onset, and enhanced stability for a controlled-release product.
Owner:HEALTH PLUS INT
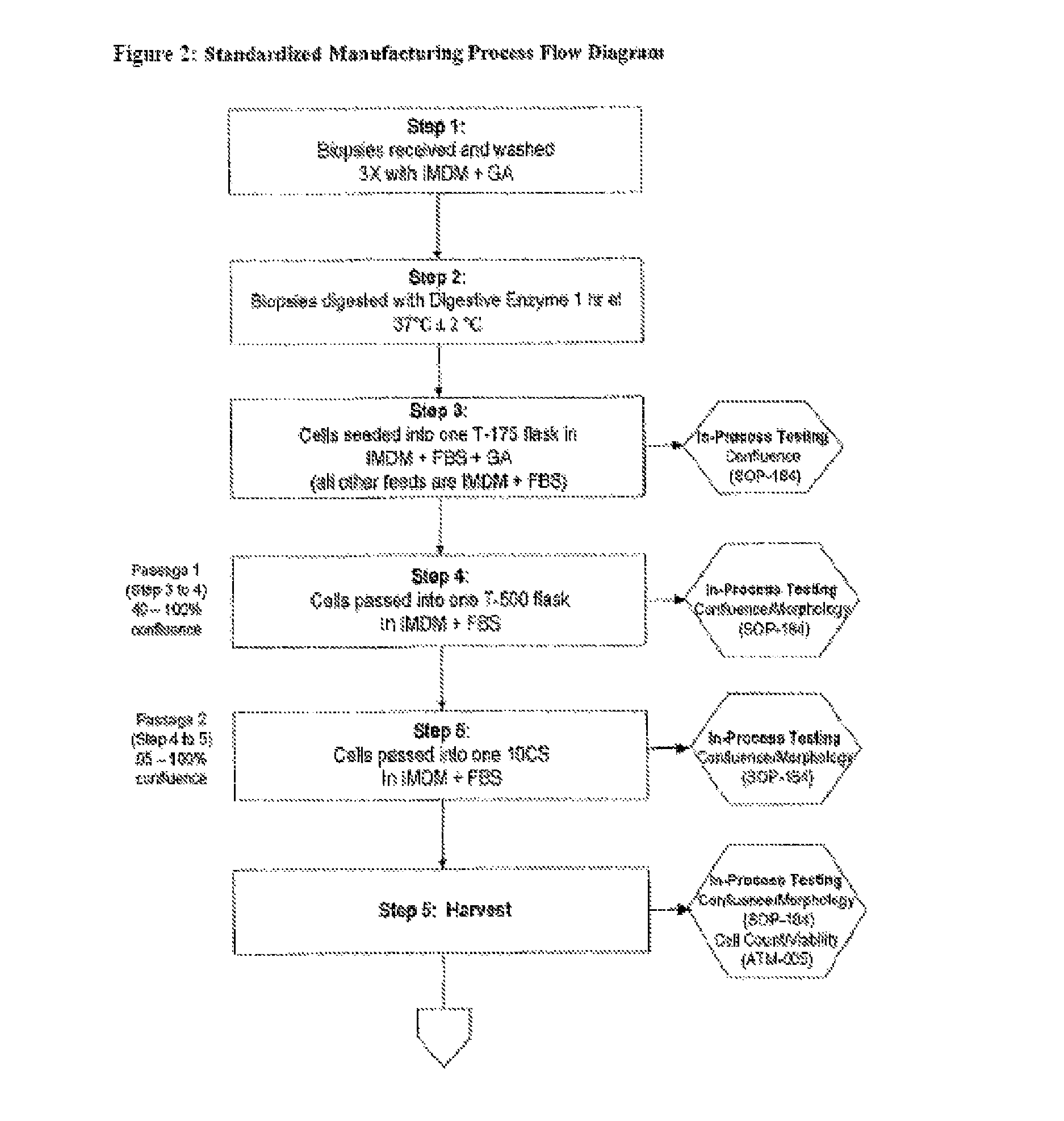
Treatment of vocal cords with autologous dermal fibroblast formulation
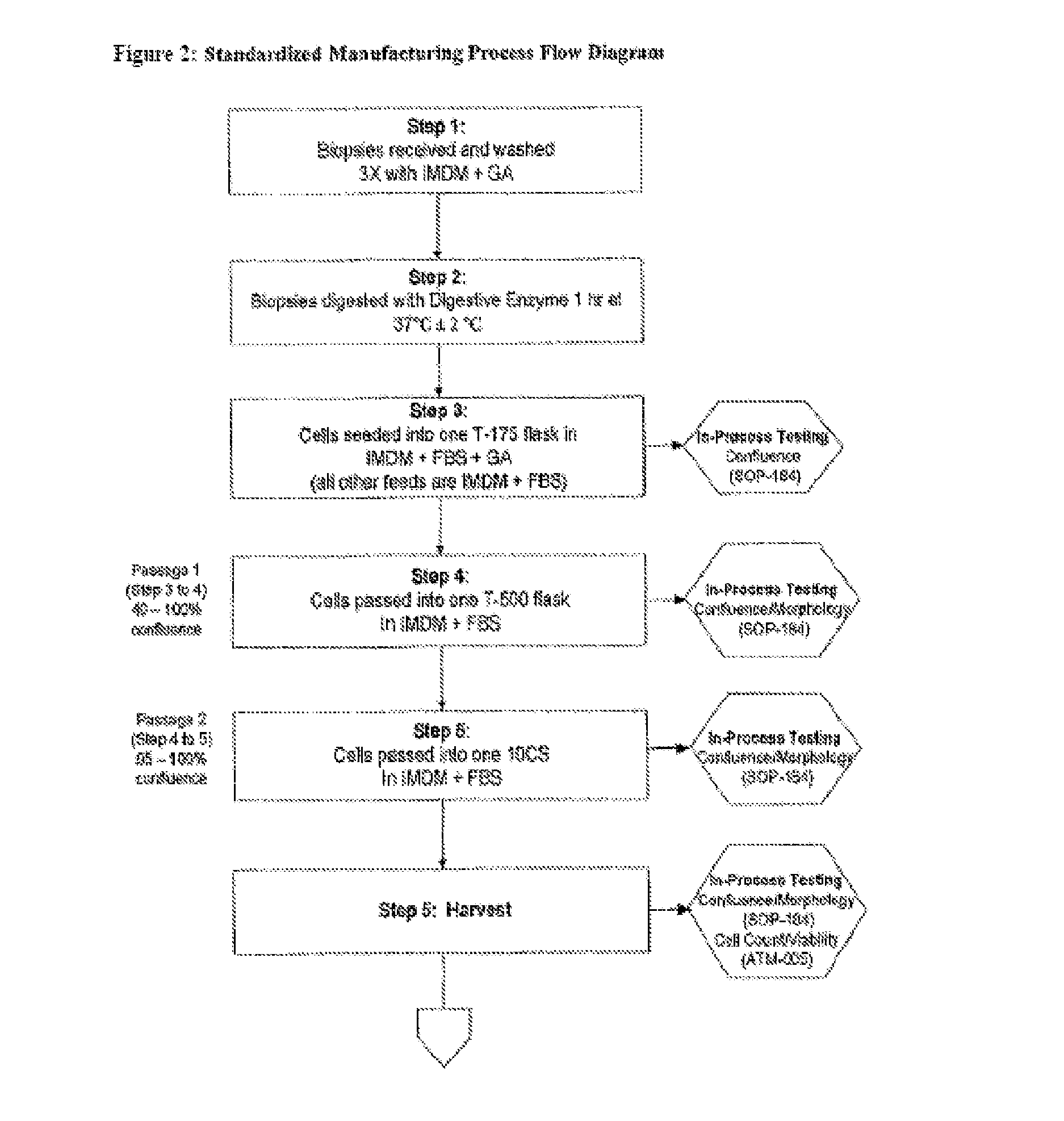
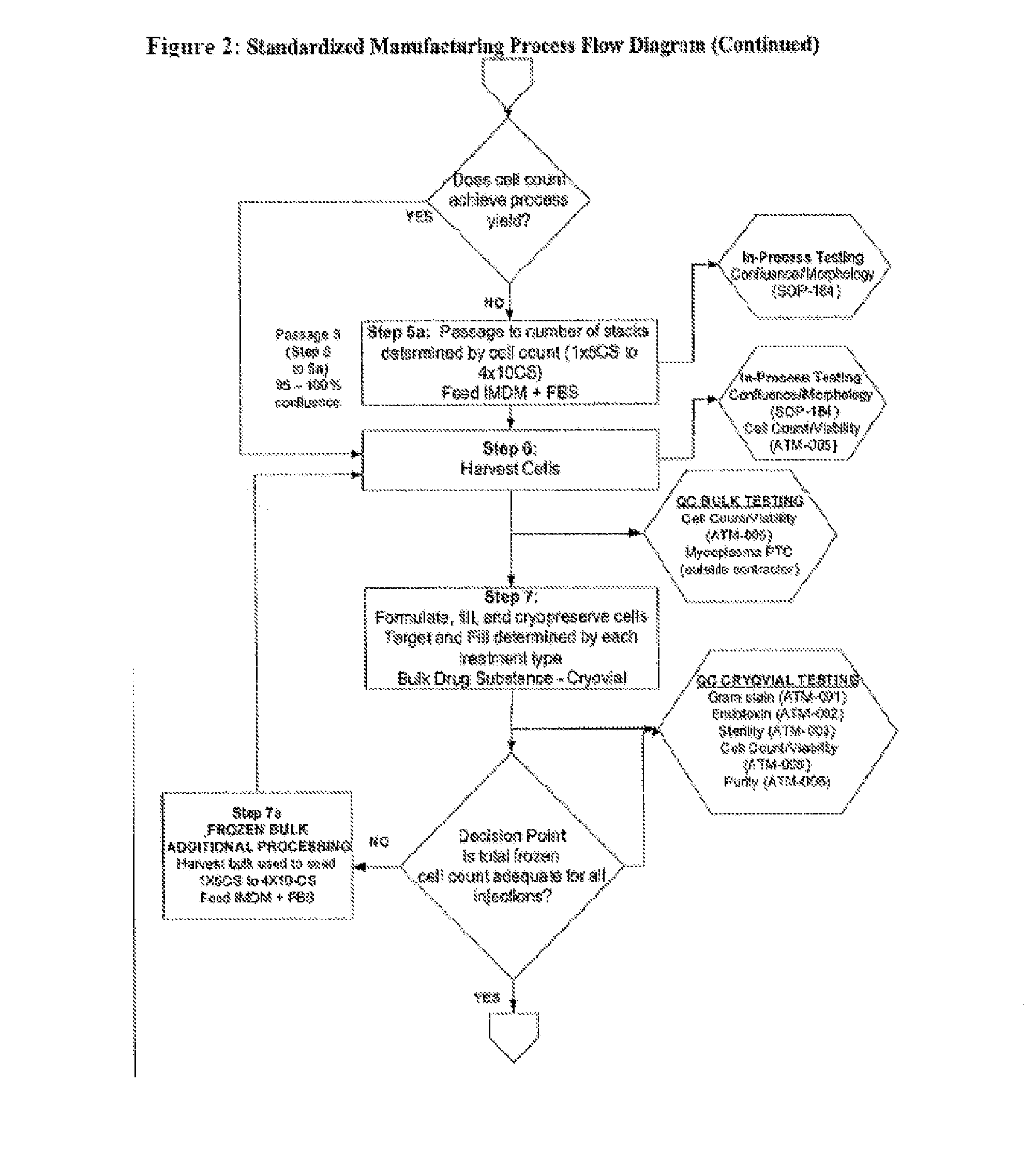
Dosage units consist of an autologous cell therapy product composed of fibroblasts grown for each individual to be treated for augmentation or regeneration of vocal cords. The suspension of autologous fibroblasts, grown from a biopsy of each individual's own buccal mucosa or skin using current good manufacturing practices (CGMP) and standard tissue culture procedures, is supplied in vials containing cryopreserved fibroblasts or precursors thereof, having a purity of at least 98% fibroblasts and a viability of at least 85%, for administration of from one to six mL, preferably two mL administered three times approximately three to six weeks apart, of cells at a concentration of from 1.0-2.0×107 cells / mL.
Owner:CASTLE CREEK BIOSCIENCES LLC
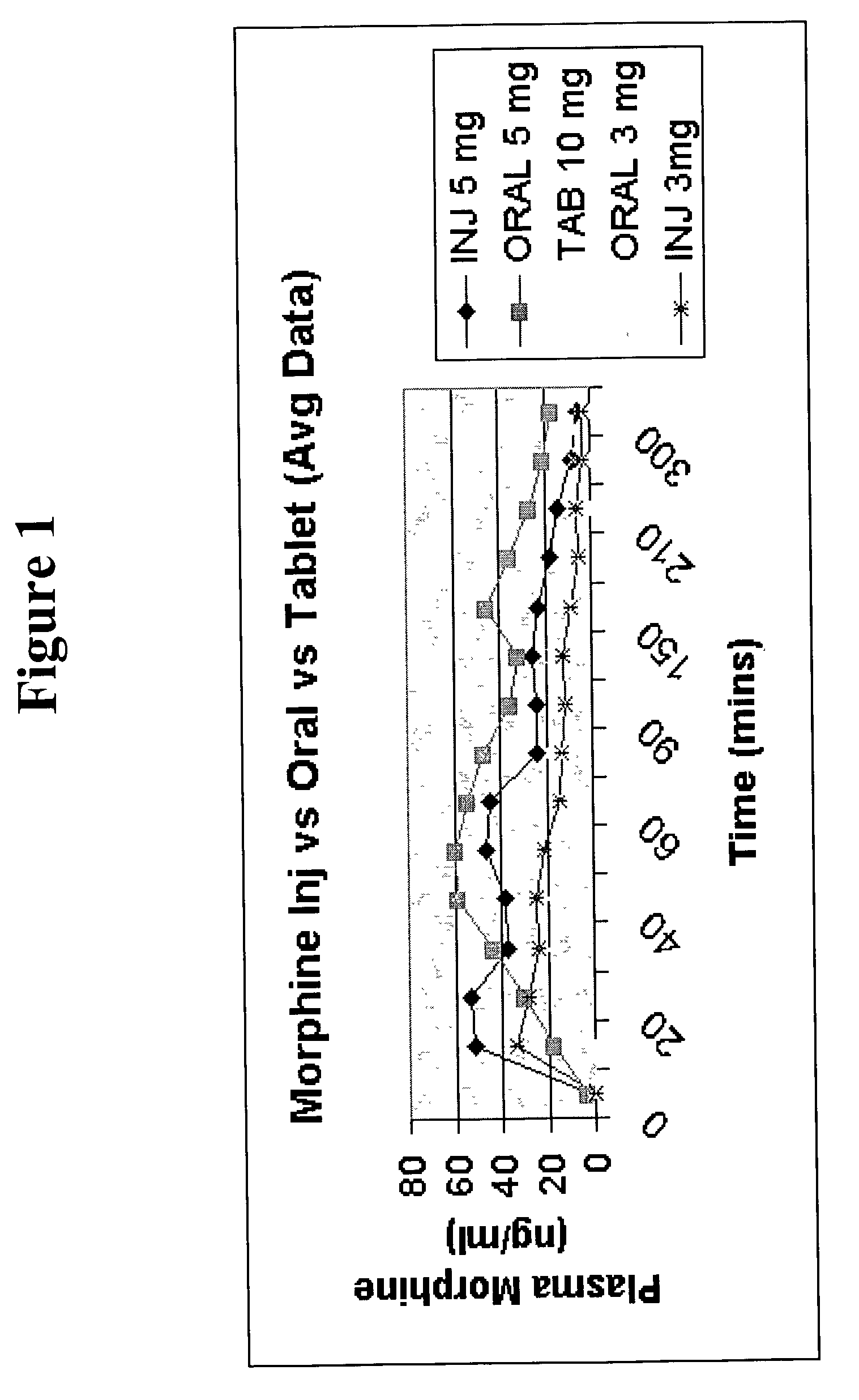
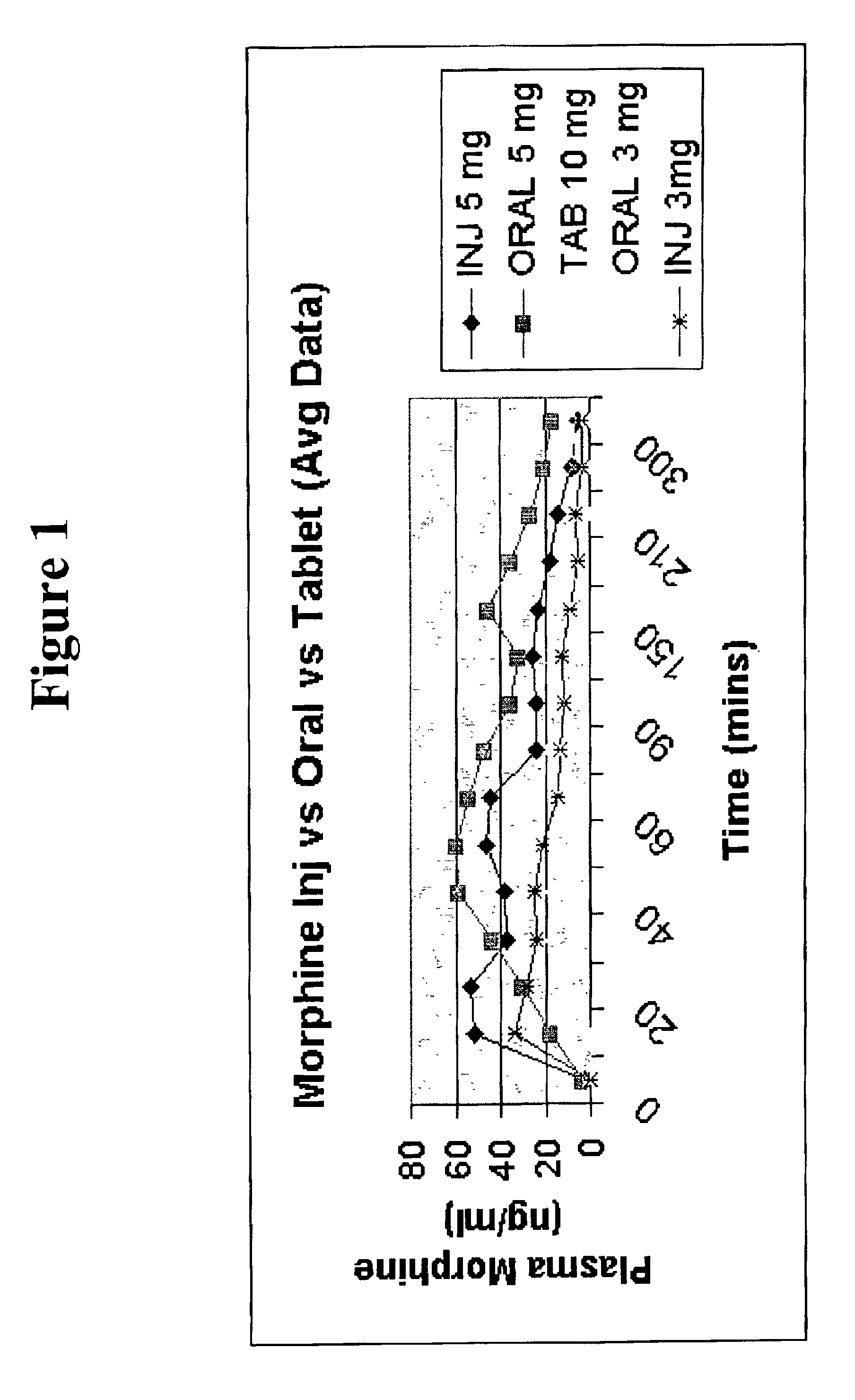
Pharmaceutical compositions for buccal delivery of pain relief medications
Pharmaceutical compositions comprising a narcotic analgesic in mixed micellar form are disclosed. The mixed micelles are formed from an alkali metal alkyl sulfate, and other micelle-forming compounds as described in the specification. Micelle size ranges between about 1 and 10 nanometers. Methods for making and using the compositions are also disclosed. A preferred method for administering the present composition is through the buccal mucosa of the mouth.
Owner:GENEREX PHARMA
Pharmaceutical compositions for buccal delivery of pain relief medications
Pharmaceutical compositions comprising a narcotic analgesic in mixed micellar form are disclosed. The mixed micelles are formed from an alkali metal alkyl sulfate, and other micelle-forming compounds as described in the specification. Micelle size ranges between about 1 and 10 nanometers. Methods for making and using the compositions are also disclosed. A preferred method for administering the present composition is through the buccal mucosa of the mouth.
Owner:GENEREX PHARMA INC
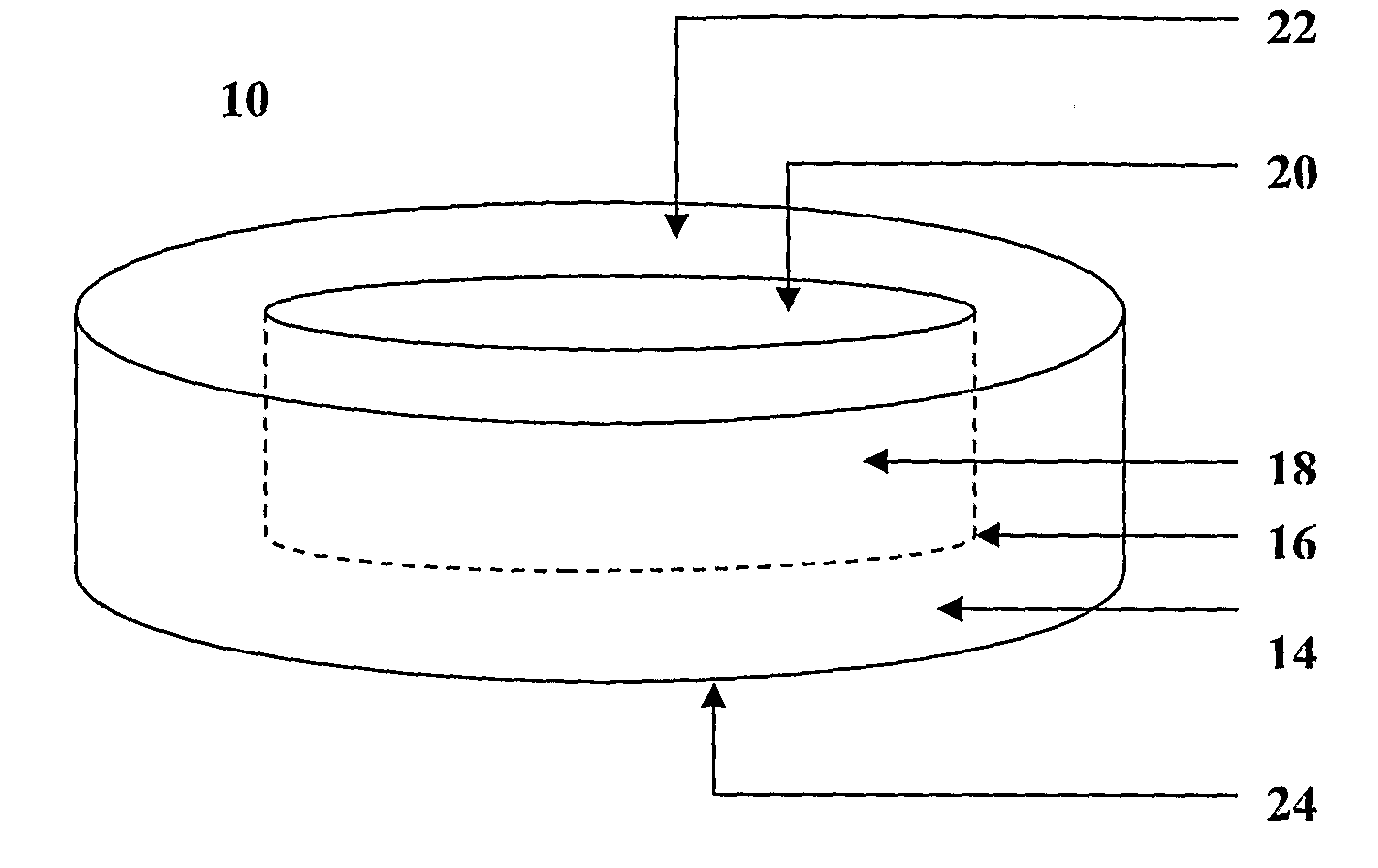
Transmucosal composition
InactiveUS20090110717A1Easy to manufactureImprove stabilityBiocidePeptide/protein ingredientsDiseaseActive agent
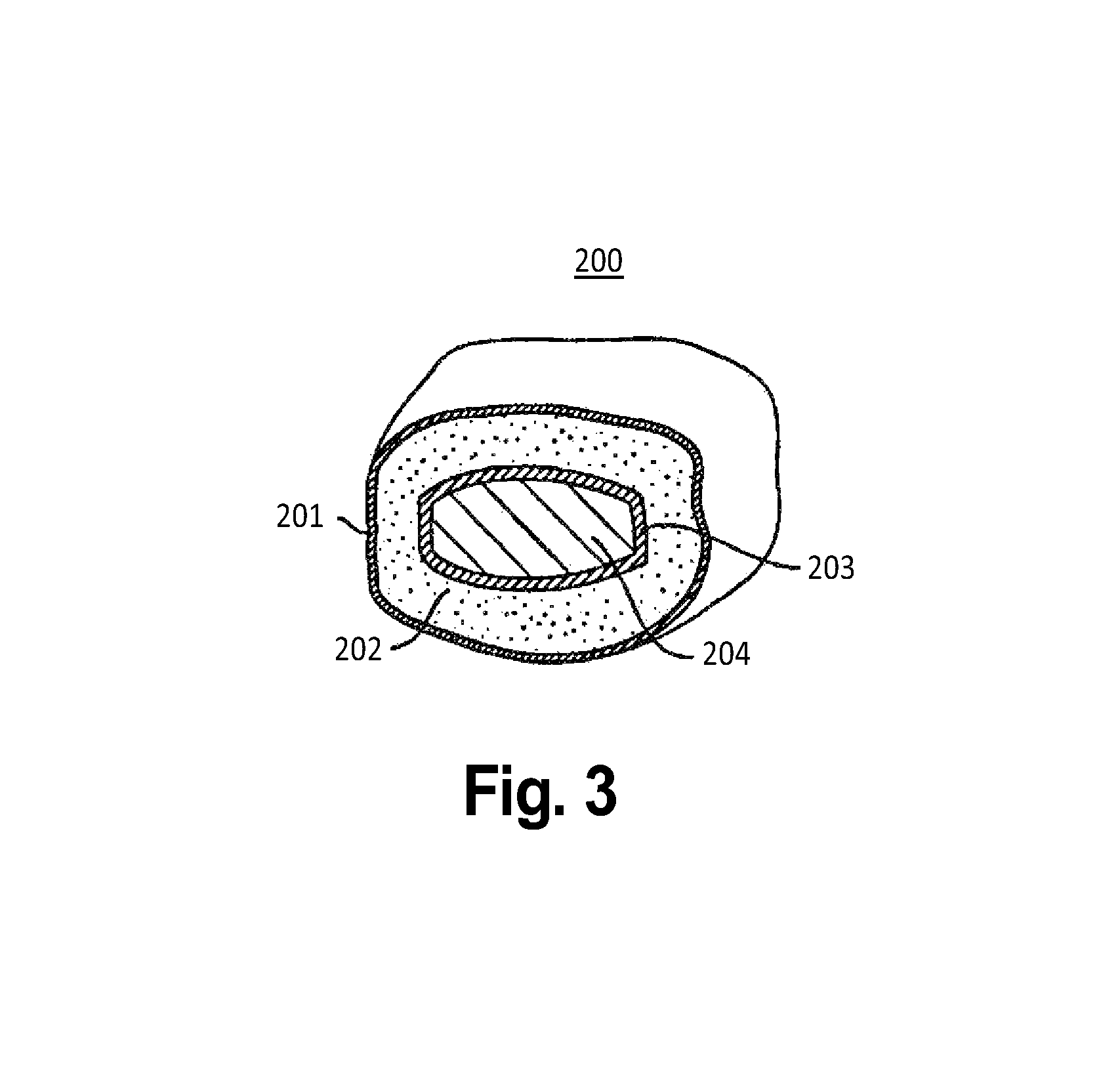
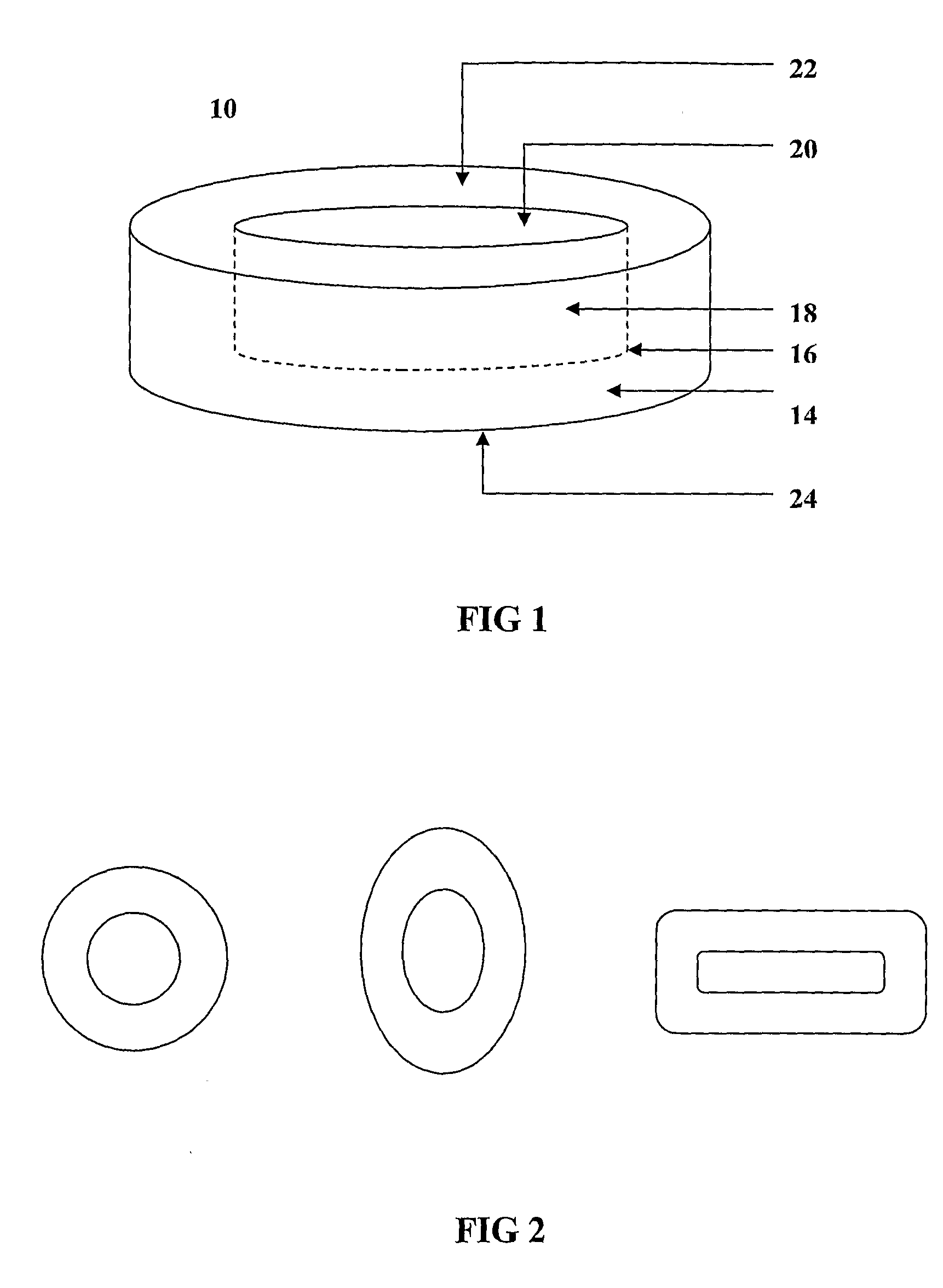
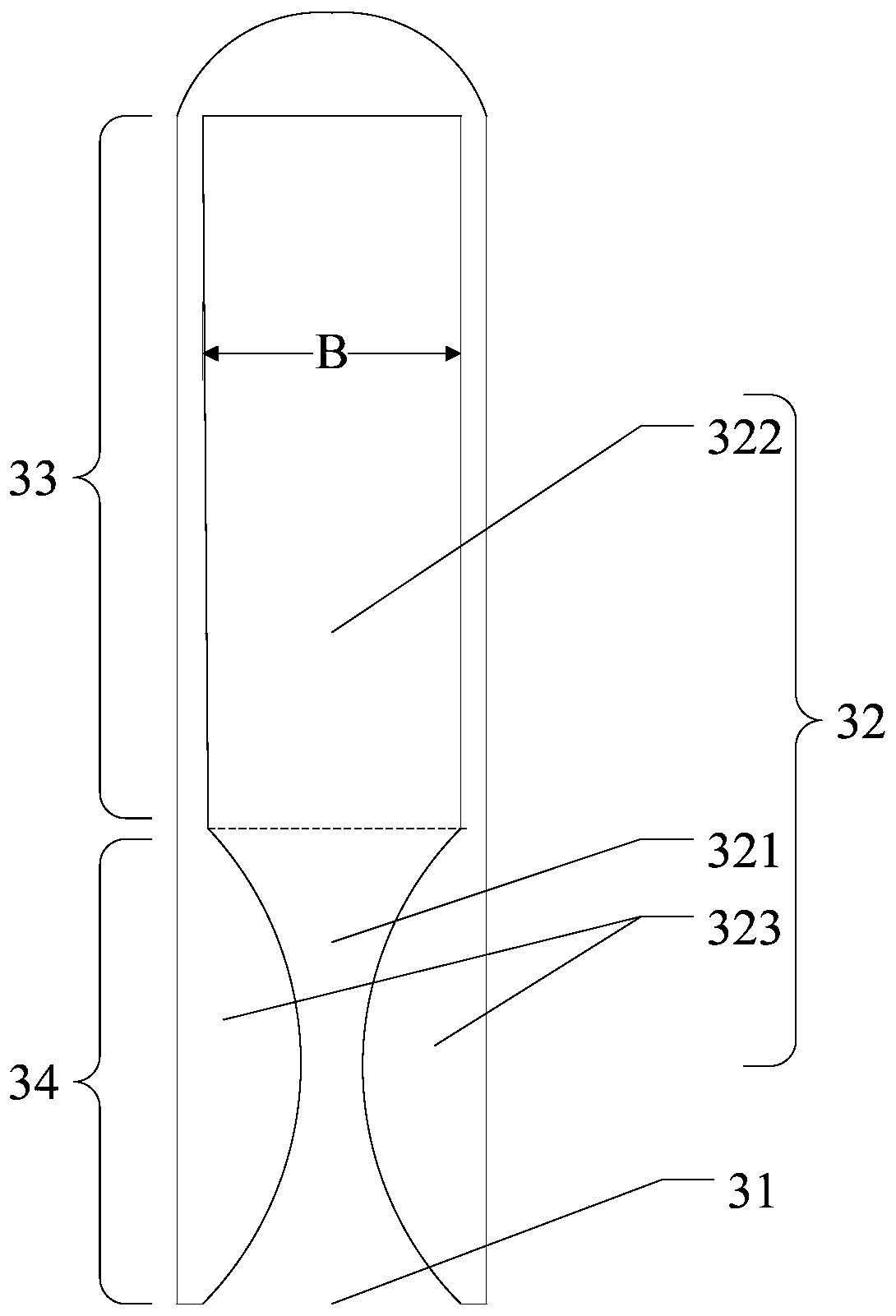
The invention provides a composition for delivering active agents through transmucosal administration, more particularly through the buccal mucosa. The composition is a unique transmucosal disk (10) which has two compartments (14) and (18); the compartments consist of at least one active agent and at least one mucoadhesive agent and both the compartments are adapted to be in contact with the mucosal membrane. The invention also provides for transmucosal administration of an active agent and method of treatment of diseases in a subject in need of such treatment.
Owner:PANACEA BIOTEC
Method for augmentation of intraepithelial and systemic exposure of therapeutic agents having substrate activity for cytochrome P450 enzymes and membrane efflux systems following vaginal and oral cavity administration
A vaginal or buccal delivery of therapeutic agents having a substrate affinity for metabolic cytochrome P-450 enzymes and membrane efflux transporter systems. A method for augmentation of systemic exposure to the therapeutic agents having a substrate affinity for cytochrome P-450 enzymes and membrane efflux transporter systems, by delivering said agents to the systemic circulation through vaginal or buccal mucosa.
Owner:FEMINA PHARMA +1
Isolation of nucleic acid from mouth epithelial cells
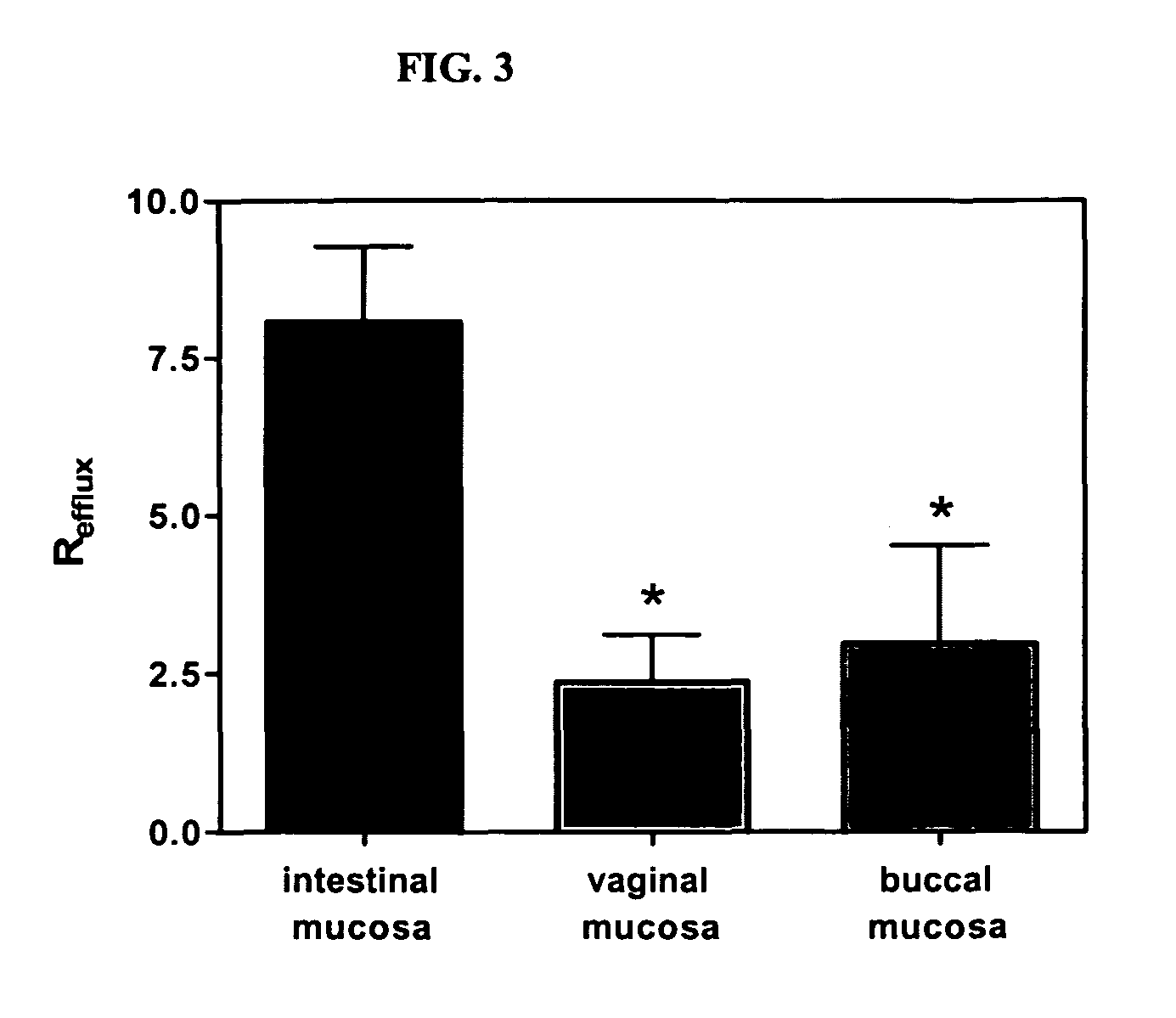
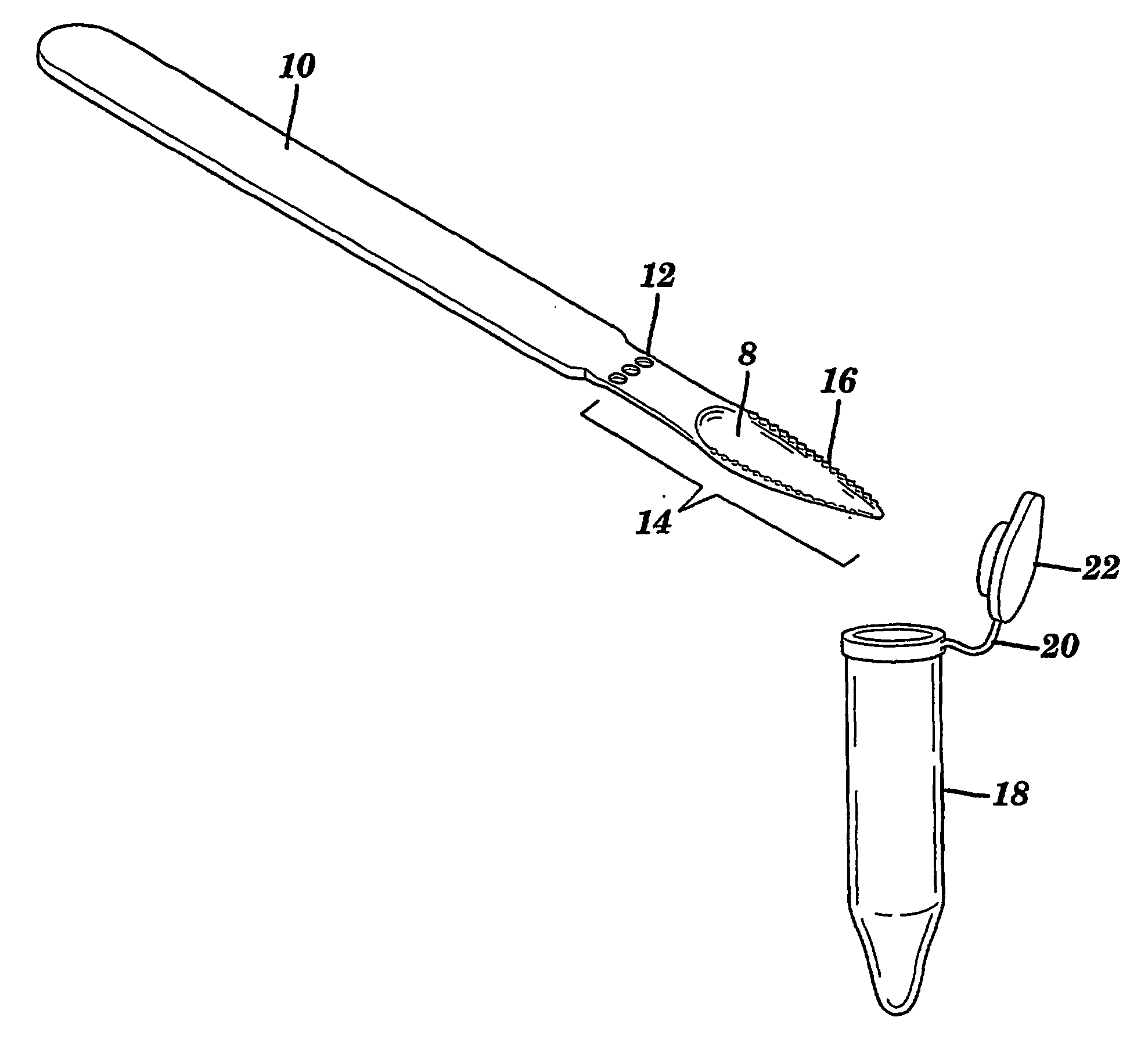
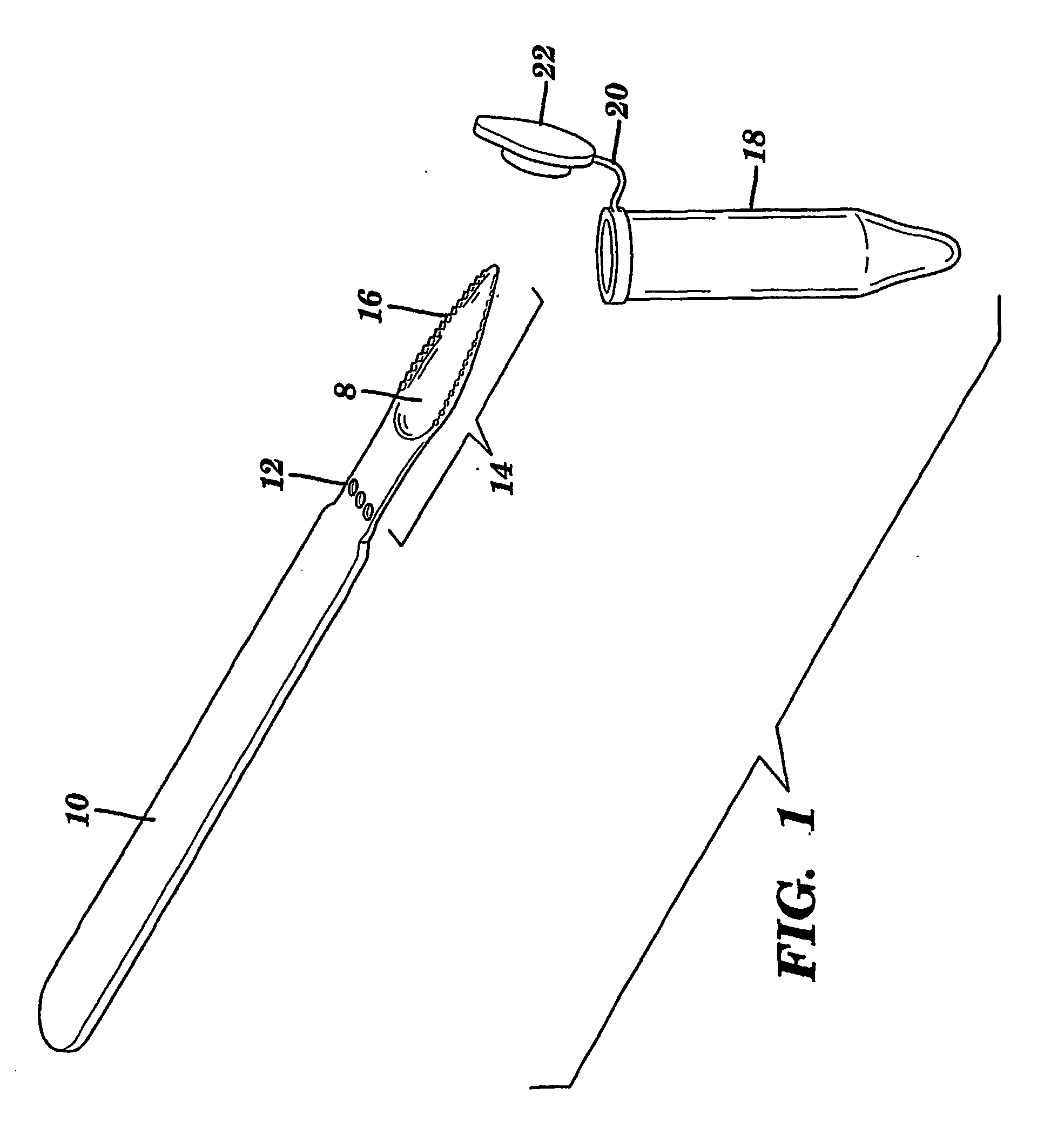
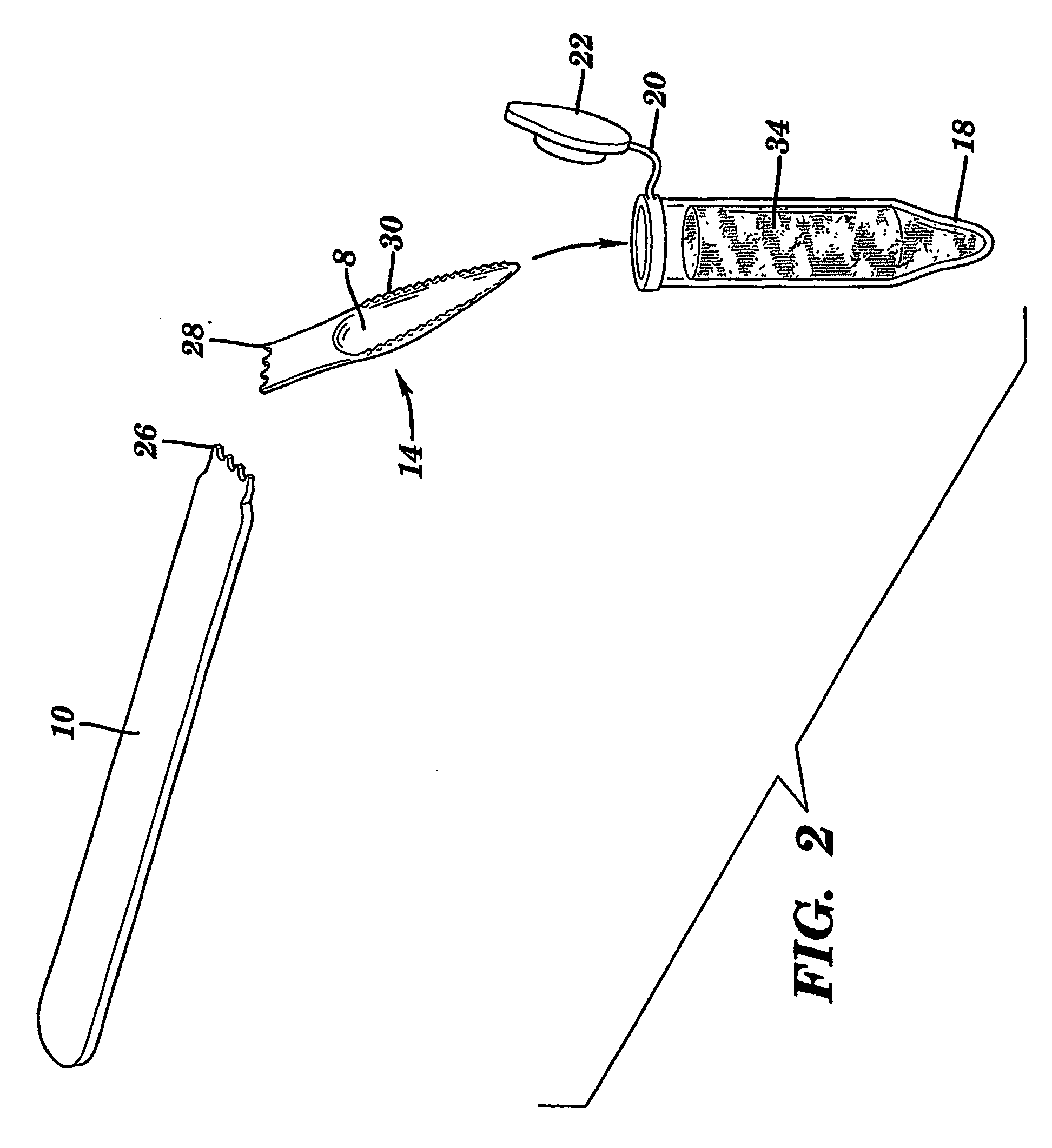
InactiveUS20070148650A1Increased riskIncreased risk of developingBioreactor/fermenter combinationsBiological substance pretreatmentsBuccal mucosaCvd risk
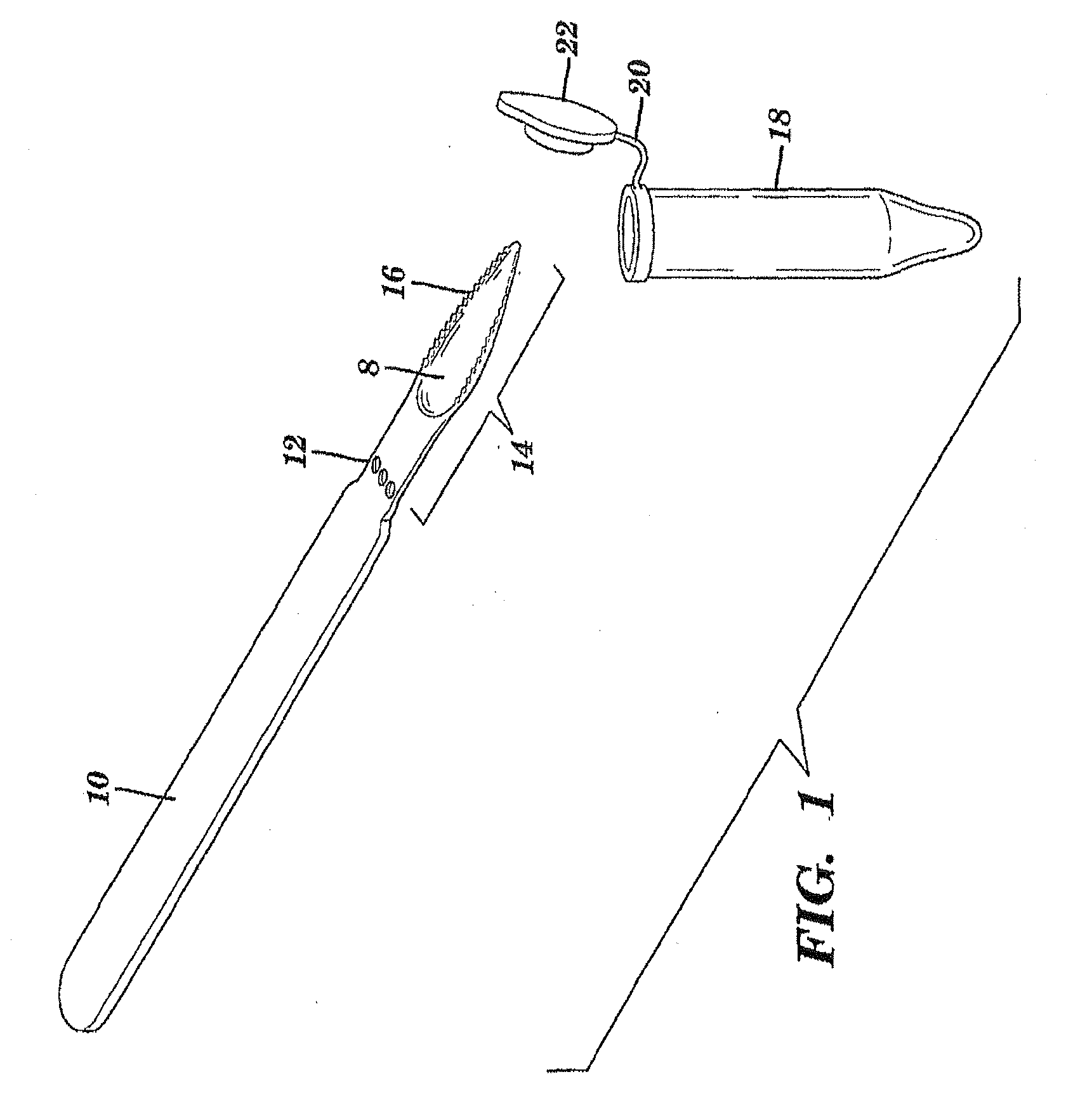
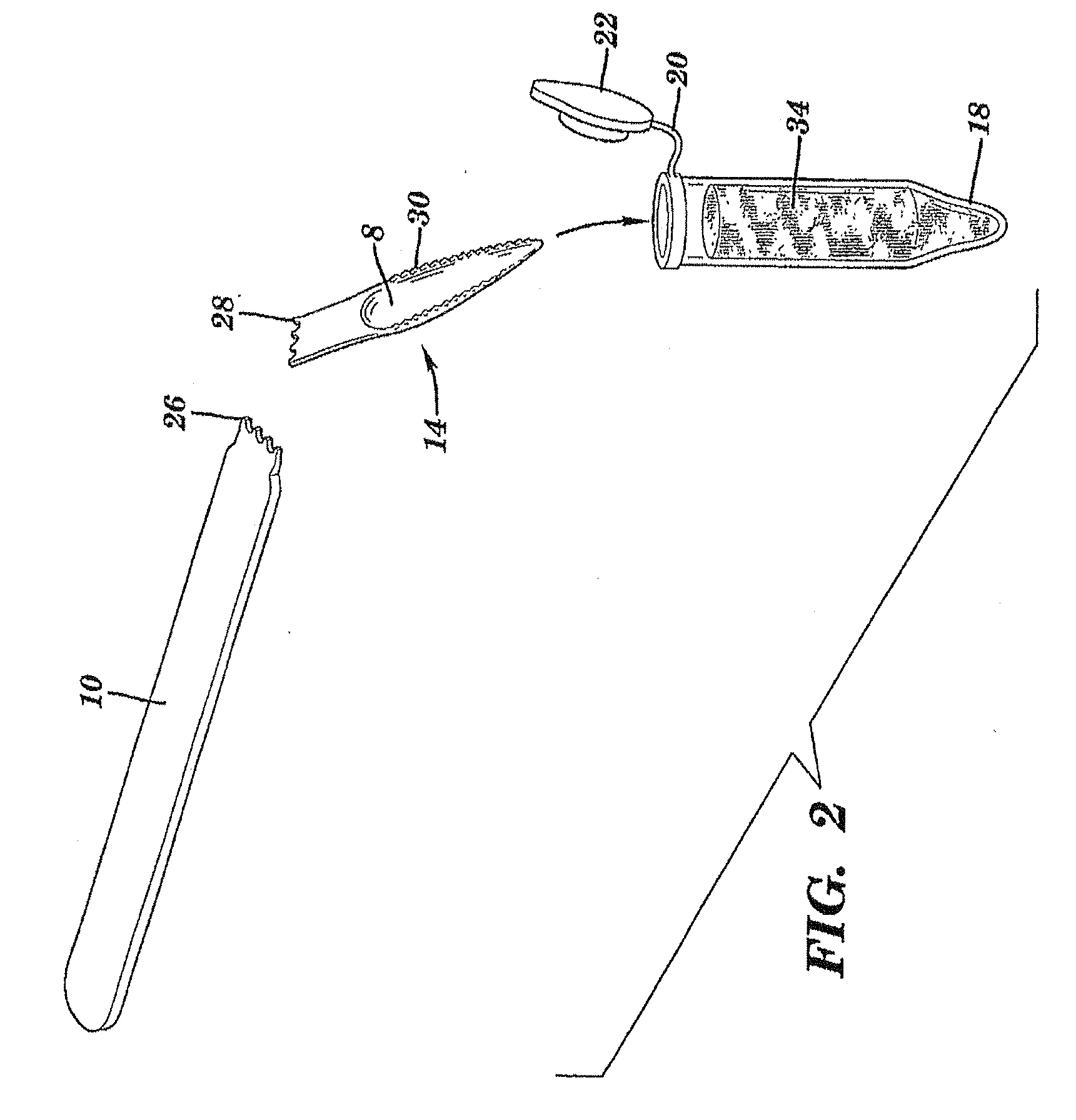
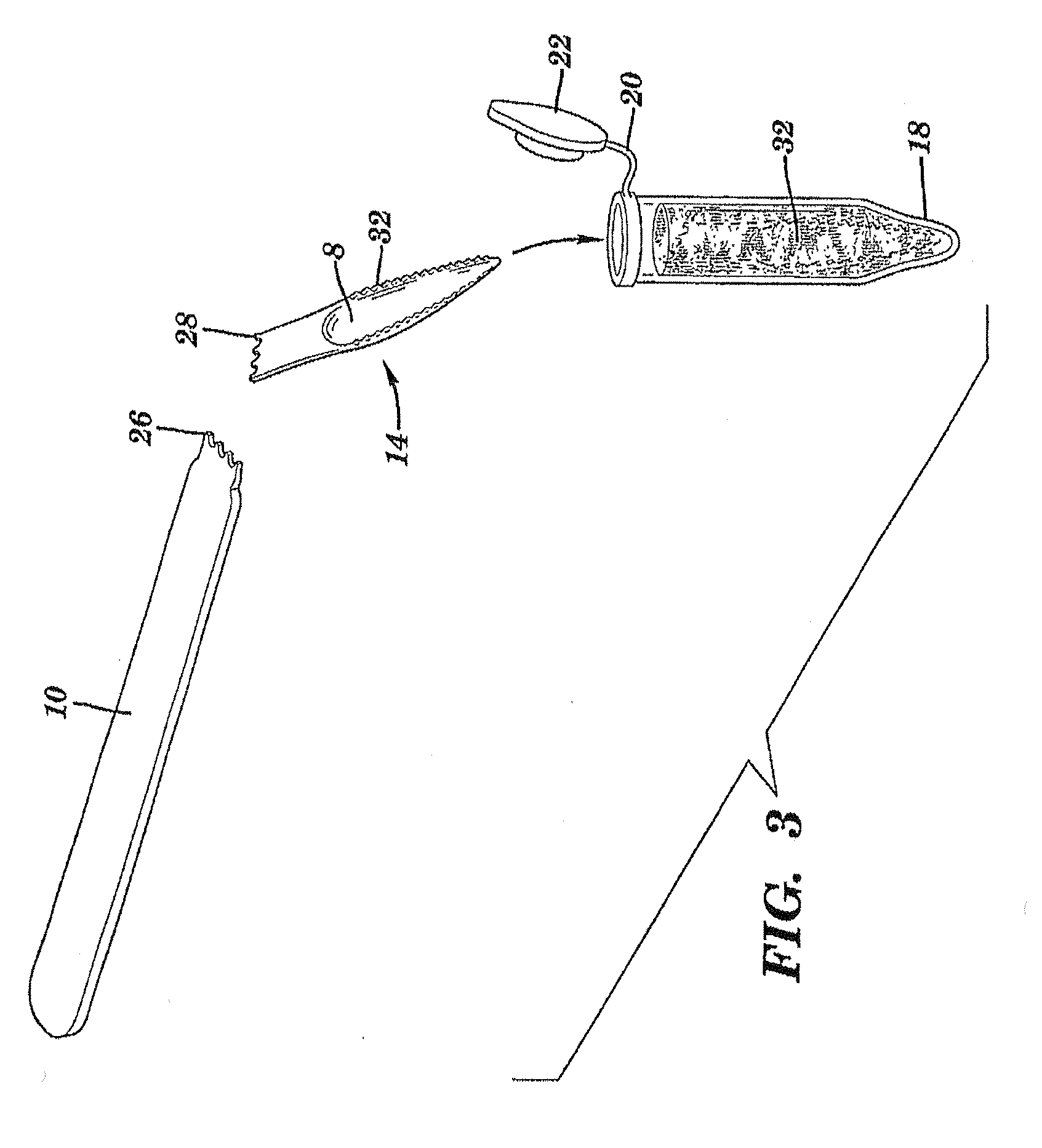
The present invention is directed to a scraping instrument for collection of a biological sample, and a non-invasive method for obtaining nucleic acid from buccal mucosa epithelial cells using the scraping instrument. Such nucleic acid can be used for example for gene expression profiling, including to assess lung disease risk associated with airway pollutants.
Owner:TRUSTEES OF BOSTON UNIV
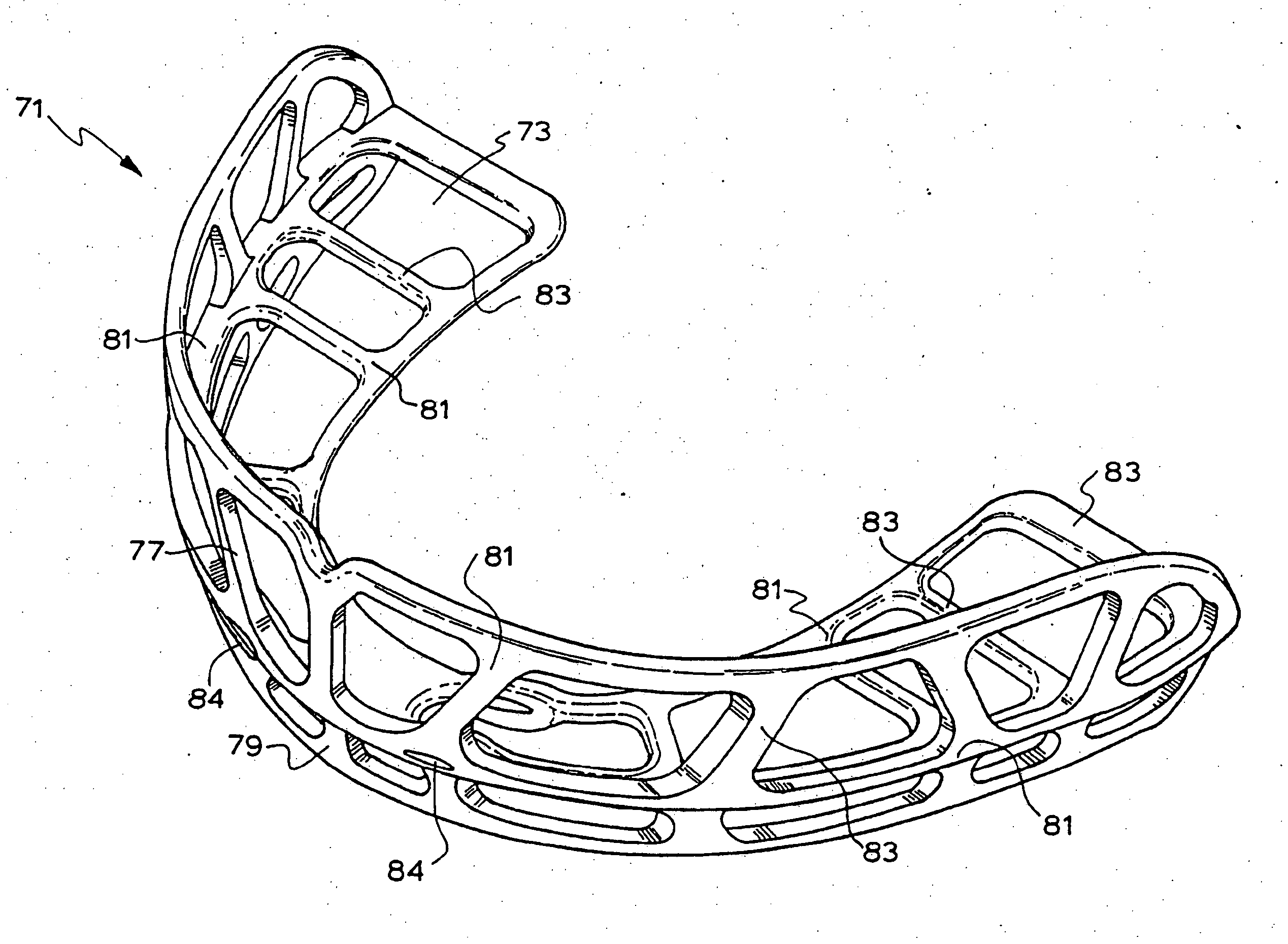
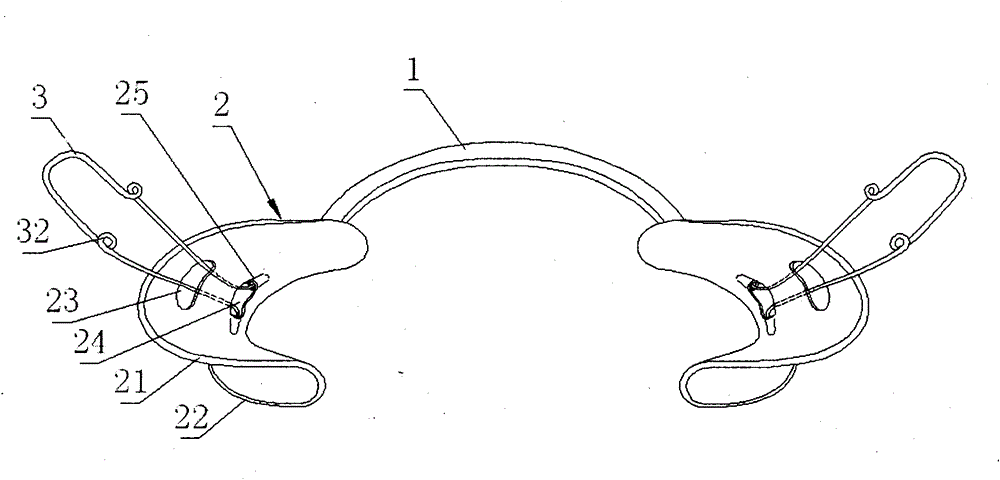
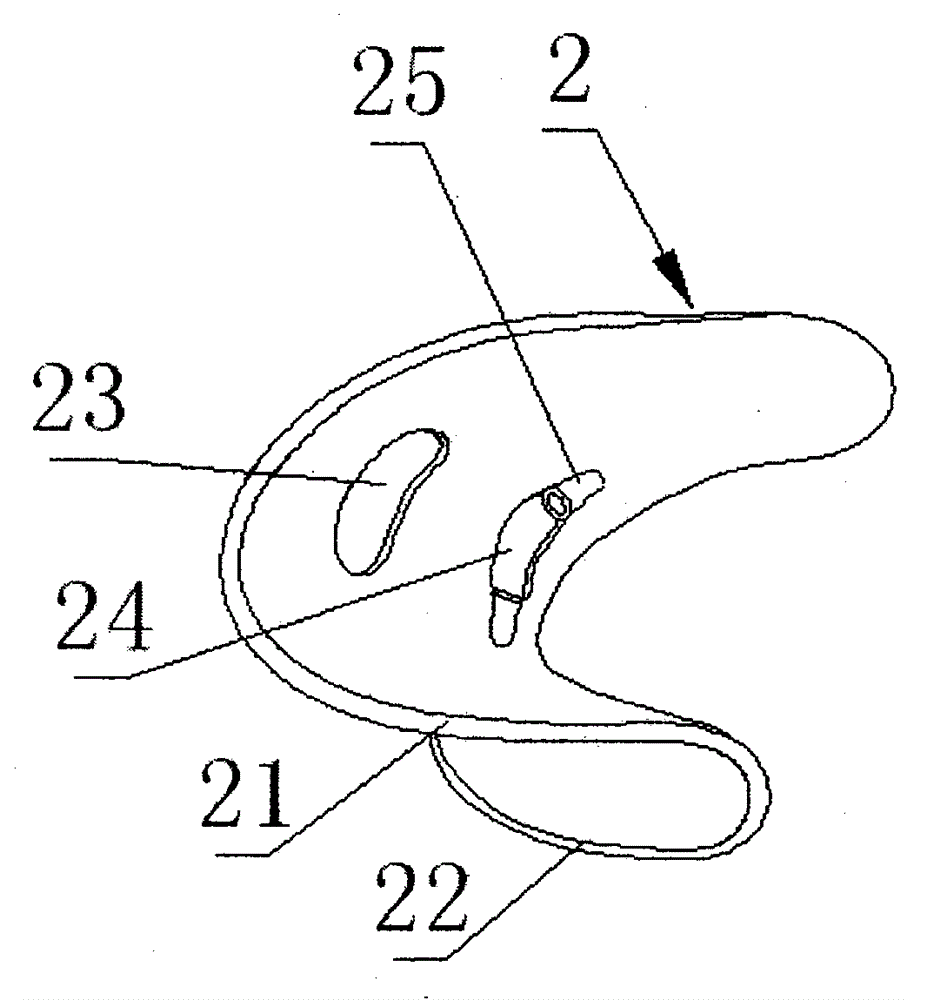
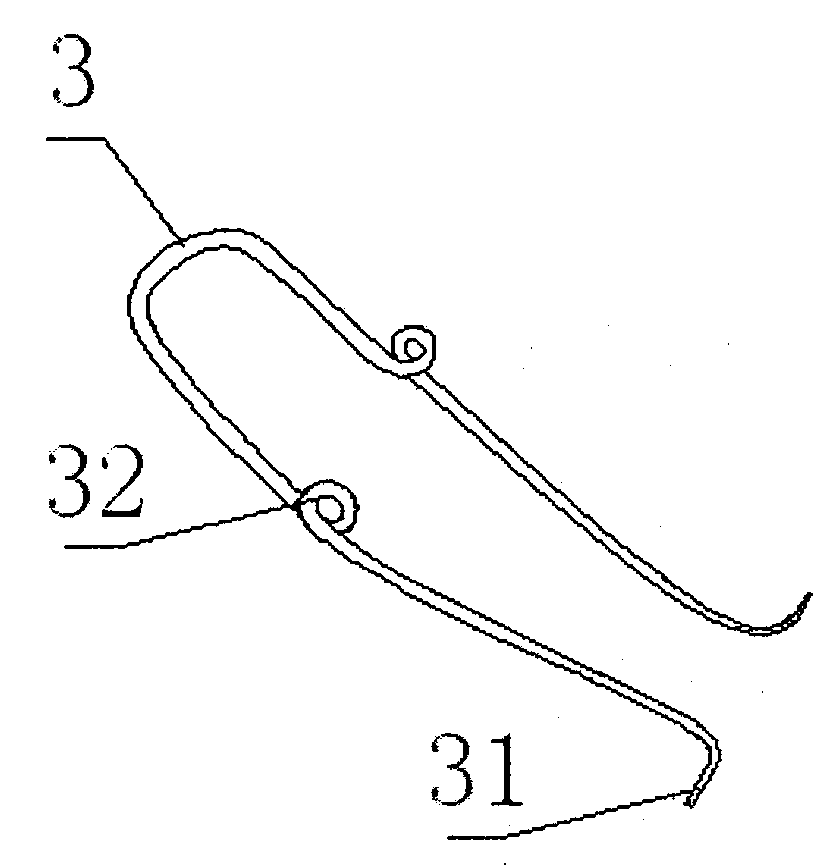
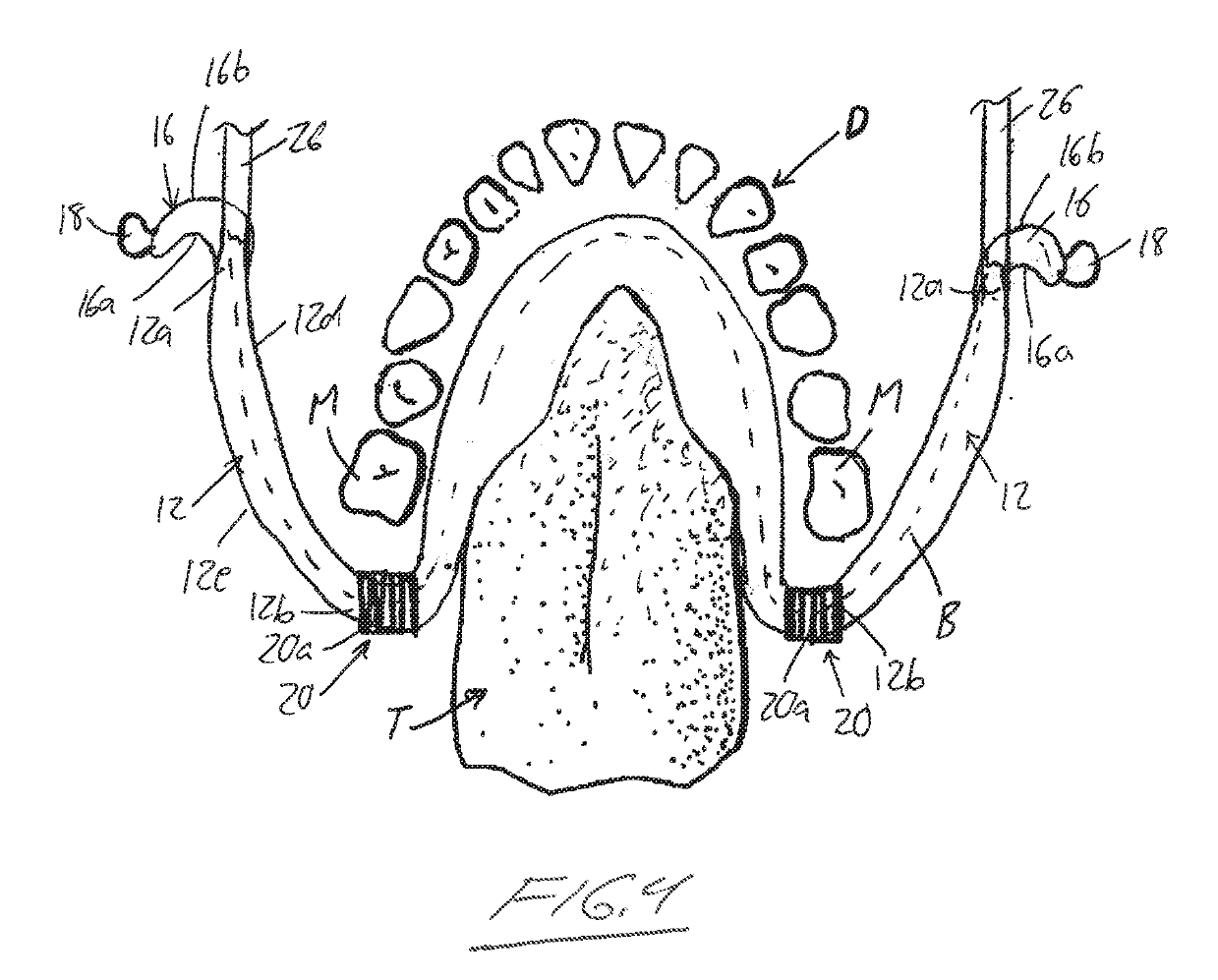
Orthodontic appliance
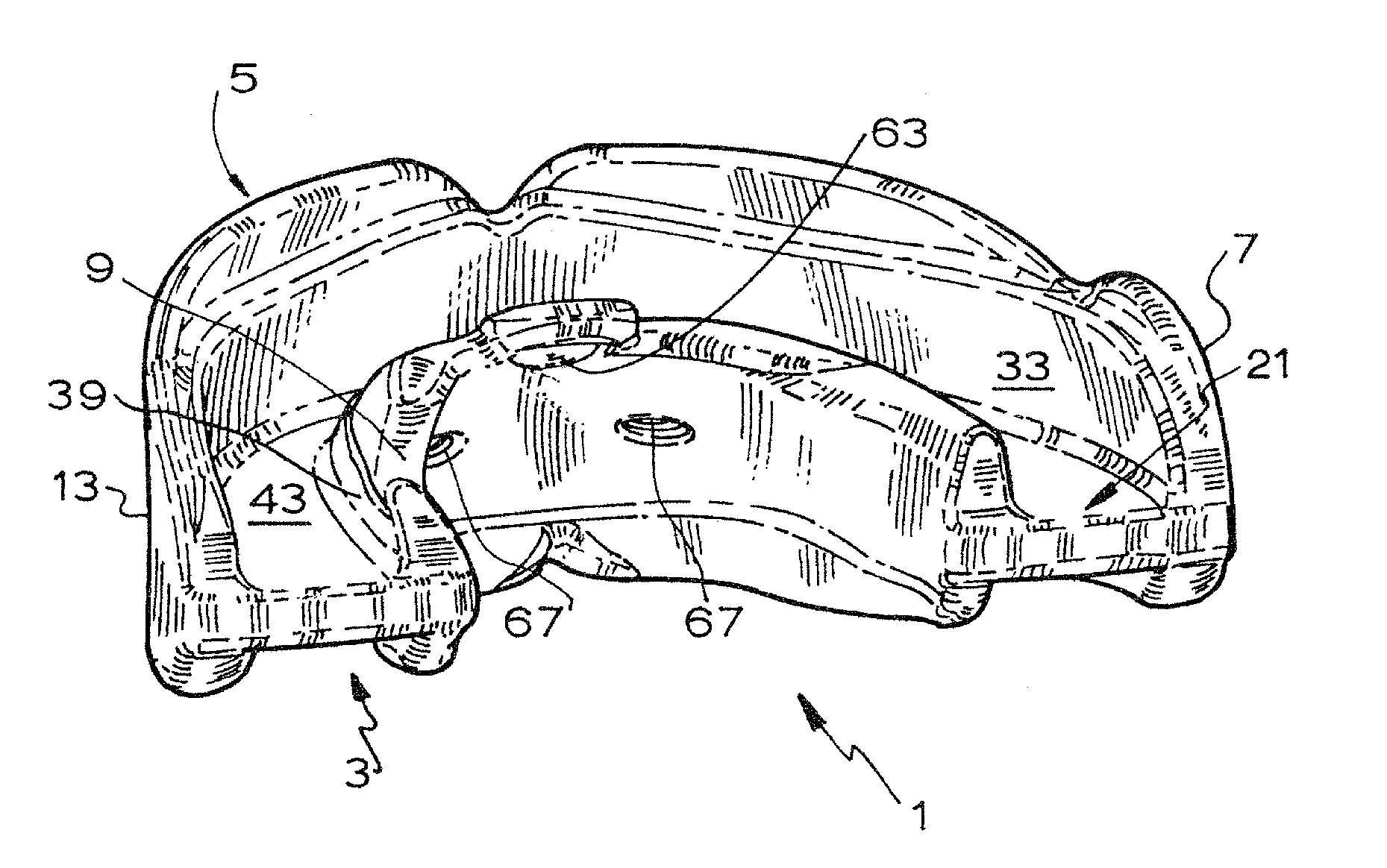
An orthodontic appliance that is particularly useful for correcting a class occlusion is disclosed. The appliance includes a mounting arrangement for mounting over an upper arch of a user having a front region and two arm regions extending rearward from the front region. The mounting arrangement includes an outer wall and an inner wall and a web extending there between. The outer wall, the web and the inner wall collectively define an upper channel within which the upper arch and teeth of a user are received to mount the appliance. They also define a lower channel for receiving the lower arch and teeth. The outer wall has an upper portion spaced forward of the upper arch teeth and gums forming an outer spacing formation for holding the buccal mucosa away therefrom. The appliance also includes a tongue elevator on a lower portion of the inner wall for raising position corresponding to an arch form will tend to return to the desired arch form. Further the resilient flexibility assists fitting of the appliance.
Owner:FARRELL CHRISTOPHER JOHN
Isolation of nucleic acid from mouth epithelial cells
InactiveUS20090311692A1Increased riskMicrobiological testing/measurementSurgical needlesBuccal mucosaCvd risk
Owner:TRUSTEES OF BOSTON UNIV
Orthodontic Appliance
An orthodontic appliance for treating a malocclusion such as a class 3 malocclusion is disclosed. The appliance comprises an outer wall and an inner wall, and a web extending between the inner and the outer walls. The inner and outer walls and the web define upper and lower channels within which the respective arches of a patient can be received. The inner and outer walls form a mounting arrangement for mounting the appliance on the upper and lower arches of the patient. The outer wall has an upper portion which is offset relative to the lower portion and the upper portion forms an outer spacing formation for holding the buccal mucosa away from the patient's upper arch so that it does not inhibit development of the upper arch. The appliance also includes a tongue elevator for lifting up the tongue to a height above the lower arch.
Owner:FARRELL CHRISTOPHER JOHN
Oral instant membrane of risperidone and preparation method thereof
InactiveCN101632651AQuick effectImprove medication complianceOrganic active ingredientsNervous disorderDifficult swallowingOrally disintegrating tablet
The invention relates to an oral instant membrane of risperidone and a preparation method thereof, thereby being used for improving the using performance of the risperidone. The technical scheme is as follows: the instant membrane comprises 2-60 parts by weight of risperidone, 25-98 parts by weight of water-soluble pharmaceutical polymer excipients, additives and 0.1-25 parts by weight of water. The oral instant membrane solves the problems that the existing risperidone is clinically difficult to swallow, is not applicable to children and elderly patients for administration and can not be conveniently taken under the situation of having no water. The oral instant membrane can be dissolved on the tongue, the hidden trouble of choking can not be happened no matter how young a baby is, the active ingredients can be absorbed sublingually and absorbed by buccal mucosa, and the onset of action is more rapid in comparison with orally disintegrating tablets. In addition, the oral instant membrane has good compliance, thereby being particularly suitable for elderly people, children and patients with difficult swallowing.
Owner:HEBEI UNIVERSITY OF SCIENCE AND TECHNOLOGY
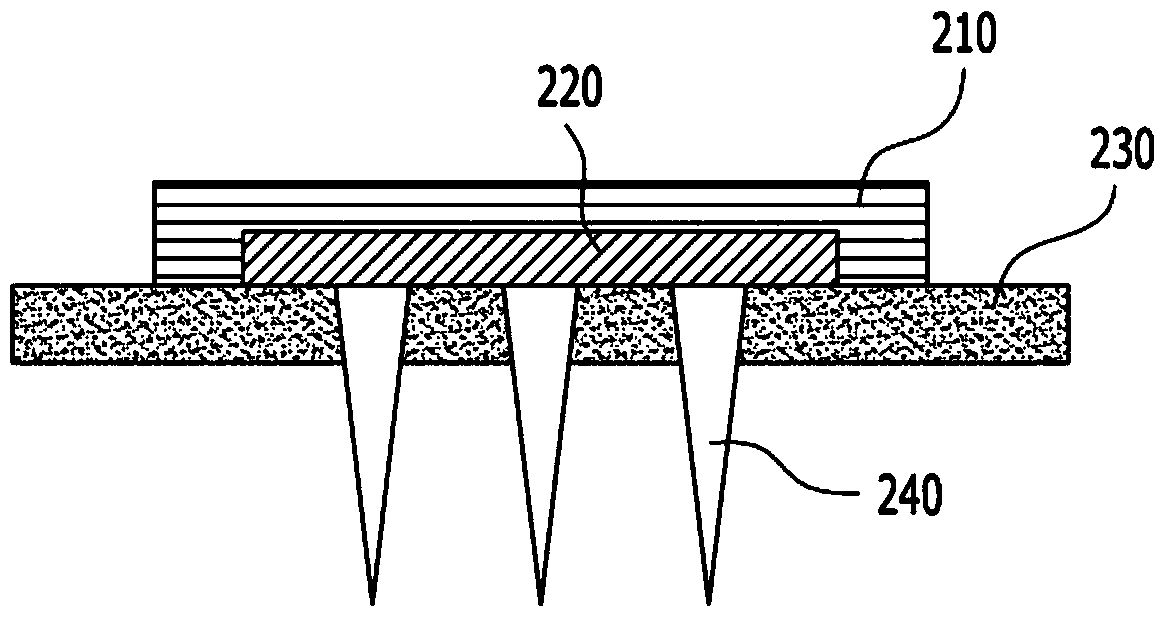
Soluble microneedle arrays for buccal delivery of vaccines
InactiveCN104185475AEasy to administerEasy and economical to makeSurgeryMicroneedlesVaccinationBuccal mucosa
A buccal microneedle patch may be provided for vaccination. The buccal microneedle patch may include at least one of microneedles. The at least one microneedles may be configured to contain a predetermined vaccine and to penetrate an outside layer of a buccal mucosa for promptly delivering the predetermined vaccine.
Owner:THERAJECT INC.
Olanzapine medicine absorbed through oral mucosa
InactiveCN102451162AEasy to industrializeIncrease productivityOrganic active ingredientsNervous disorderIrritationAdhesive
The invention relates to an olanzapine medicine absorbed through an oral mucosa. The medicine is composed of olanzapine taken as an effective medicinal component and auxiliary components acceptable in oral mucosa medicines, wherein, the auxiliary components comprise a disintegrant, a filling agent, a flavoring agent, an adhesive, a mucosa absorption enhancer, and a lubricant. Specifically, the medicine includes the following preparation forms employed currently: sublingual tablets, toroches, buccal tablets, buccal adhesive tablets, and buccal patches, etc. Able to be completely or mainly absorbed by a sublingual mucosa and / or a buccal mucosa, the medicine of the invention has the advantages of no irritation on the oral cavity, ability to substantially improve medicinal bioavailability and treatment effect, as well as convenient administration, and can bring patients good compliance.
Owner:重庆市力扬医药开发有限公司
Palonosetron oral transmucosal film or patch
ActiveUS9937122B2Easy to receiveQuick releaseOrganic active ingredientsDigestive systemMentholPlasticizer
The present invention provides a pharmaceutical composition for delivering palonosetron through the buccal mucosa or sublingual mucosa. The pharmaceutical composition comprises 0.05-35% (w / w) of palonosetron, 40-90% of a film forming agent, 1-10% (w / w) of a plasticizer, 5-25% (w / v) of an adhesive agent, and 0.1-5% of a penetration enhancing agent. A preferred plasticizer is a polysorbate. A preferred adhesive agent is polyvinylpyrrolidone or carboxymethylcellulose. A preferred penetration enhancing agent is peppermint oil or menthol.
Owner:LP PHARM (XIAMEN) CO LTD
Novel keratoprosthesis, and system and method of corneal repair using same
ActiveUS20150216651A1Improved soft tissue adhesionLess squeezeEye implantsAcid etchingAnterior cornea
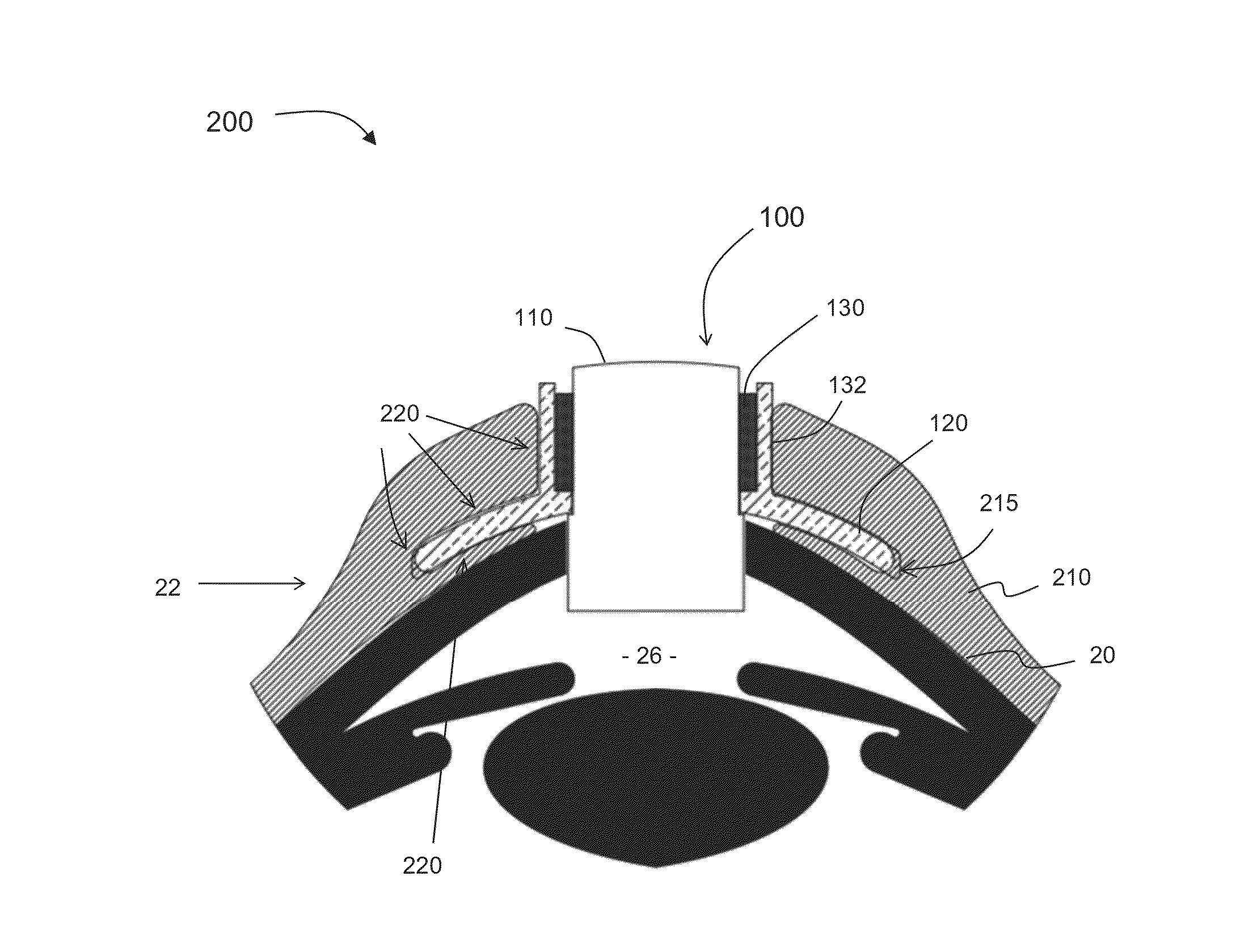
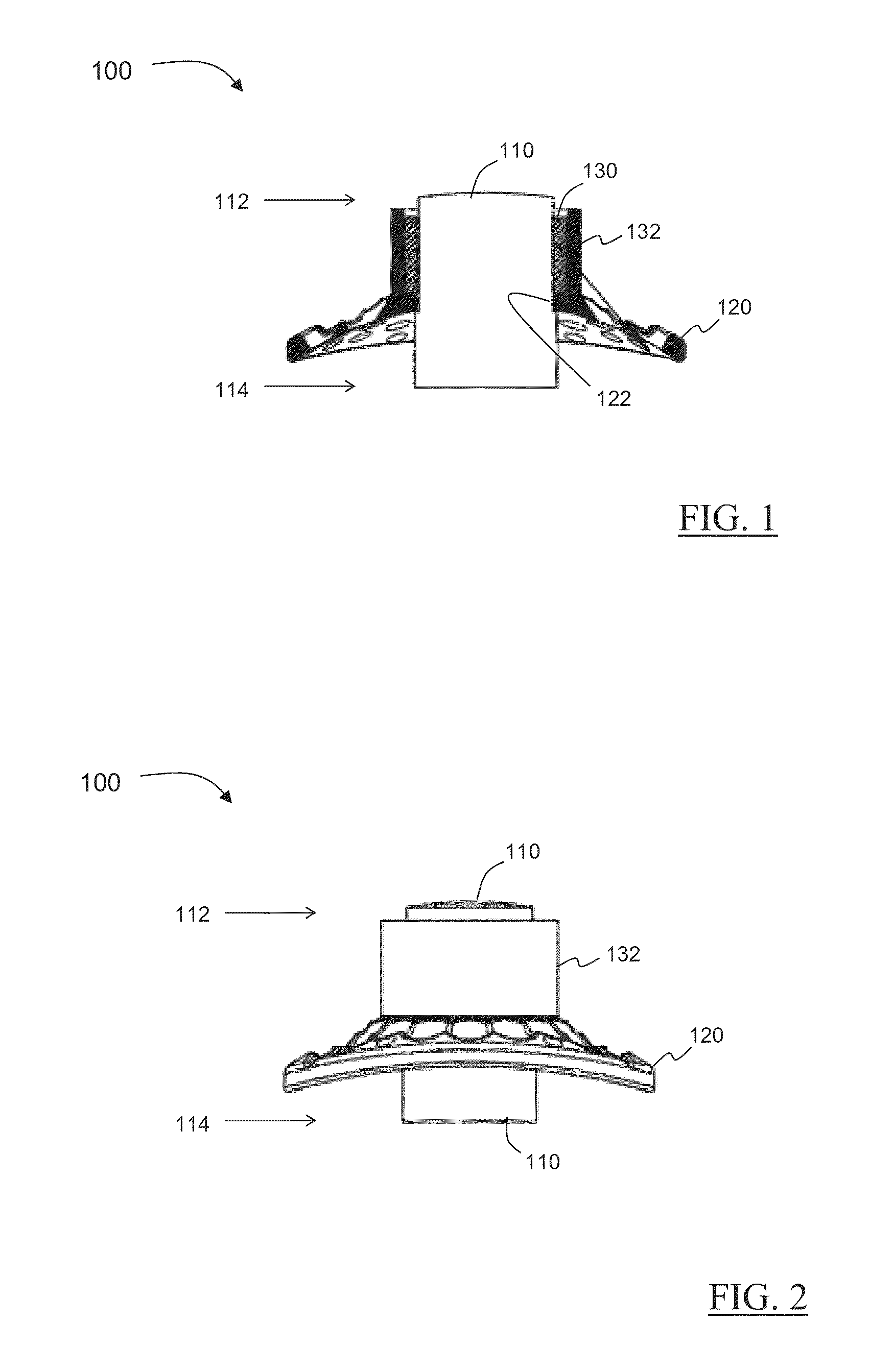
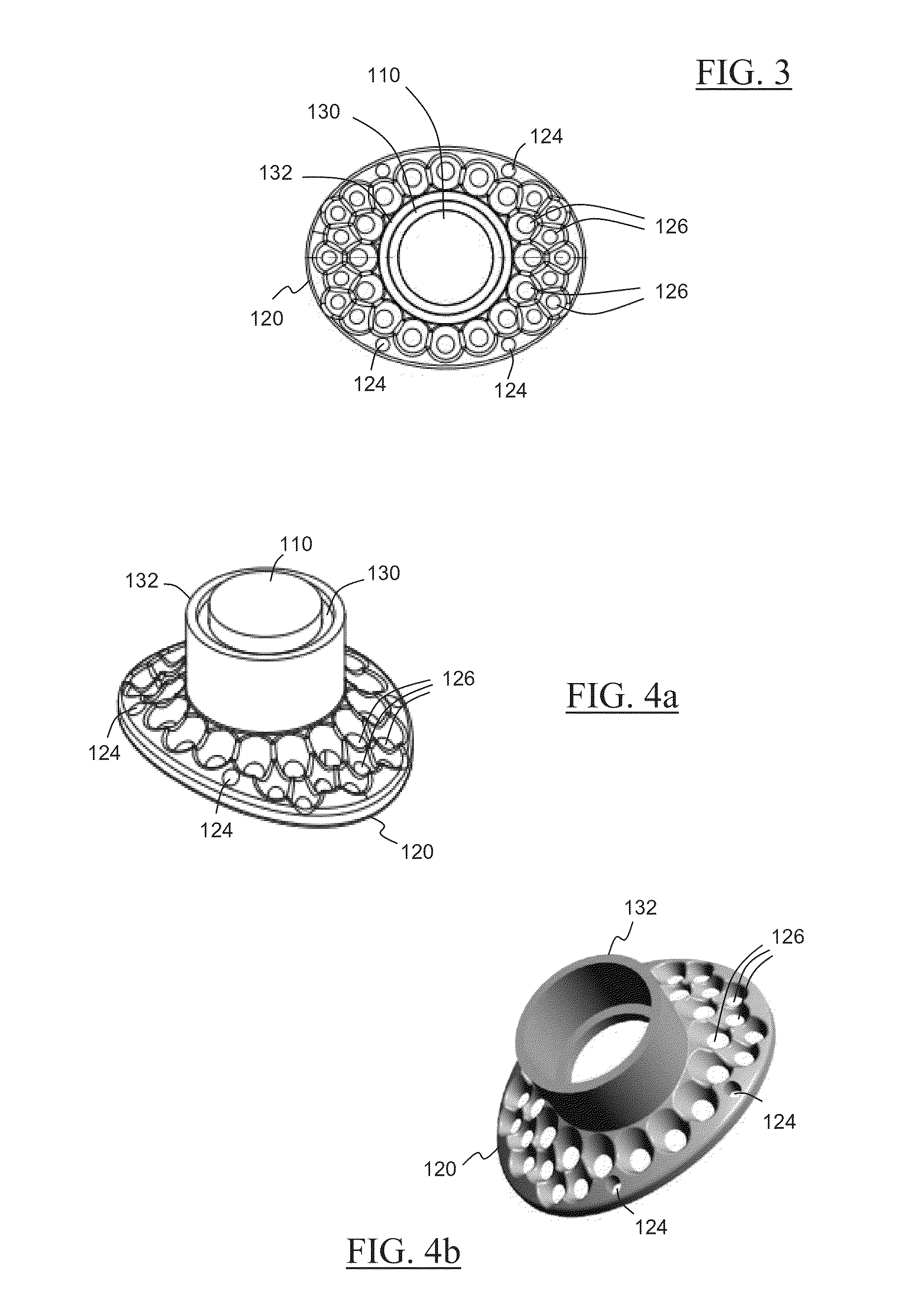
A keratoprosthesis and system and method of using same for corneal repair. The keratoprosthesis comprises a biocompatible support and an optic member disposed through a channel within the support. The support includes metal, preferably titanium, and treated, such as by sandblasting and / or acid etching, to create textured surfaces that promote soft tissue adhesion. A locking member interconnects the optic member and support. An outer surface of the locking member a collar extending from the support and disposed around the optic member is also metal, preferably titanium, and is similarly treated to promote soft tissue adhesion. A locking member interconnects the optic member and support. The system includes the keratoprosthesis positioned within an isolated soft tissue segment of a non-ocular tissue, such as buccal mucosa, placed on the anterior cornea. The method includes removing corneal epithelium, isolating and transplanting a segment of soft tissue to the de-epithelialized cornea, creating a receiving area in the soft tissue, positioning a keratoprosthesis relative to the receiving area anterior to the cornea, and securing the keratoprosthesis.
Owner:UNIV OF MIAMI
An adjustable orthodontic mouthpiece
The invention discloses an adjustable orthodontic mouth gag comprising an oral cavity front part supporter and adjustable molar area support wings, wherein the oral cavity front part supporter comprises a connecting rod and two auricles; each auricle consists of an auricle large wing and an auricle small wing; a positioning hole I and a positioning hole II are formed in the auricle large wing, and a positioning pipe is arranged on each of two opposite sides of the positioning hole II; each of the adjustable molar area support wings is an elastic U-shaped rod, and a strength adjusting spring is arranged on each of two sides of the elastic U-shaped rod; one elastic U-shaped rod is arranged on each of the auricles; the two ends of each elastic U-shaped rod penetrate into the positioning hole II, and are clamped into the corresponding positioning pipe; the middle part of each elastic U-shaped rod extends to the outer side of the auricle large wing. According to the adjustable orthodontic mouth gag disclosed by the invention, the adjustable molar area support wings are arranged on the auricles to unfold the buccal mucosa of the molar area, so that the binding of orthodontic attachments of a posterior tooth area is facilitated; the supporting strength of the buccal mucosa of the molar area can be adjusted, the operations can be facilitated, and the discomfort in the operation of the mouth gag can be minimized at the same time.
Owner:THE SECOND AFFILIATED HOSPITAL OF CHONGQING MEDICAL UNIV
Oral protection mat suitable for oral treatment and using method thereof
PendingCN108524044AFacilitate passive openingRelieve masseter muscle fatigueSurgerySomatoscopeOral treatmentBuccal mucosa
The invention discloses an oral protection mat suitable for oral treatment. The oral protection mat comprises a buccal mucosa protective baffle plate, a retromolar tissue protective baffle plate and atongue protective baffle plate, wherein a circle of water draining pipelines are arranged at the periphery of the tongue protective baffle plate in a surrounding way, and 6 to 8 liquid sucking holesare formed in the tongue protective baffle plate; two sides of the water draining pipelines respectively stretch outwards along a horizontal direction to form skirts; the tongue protective baffle plate is provided with a negative-pressure interface converter; one end of the negative-pressure interface converter is communicated with the water draining pipelines, and the other end is communicated with a negative-pressure pipe of an oral integrated treatment table in a joint buckling connection way. The invention also relates to a using method of the oral protection mat for the oral treatment. The using method is simple and easy, is capable of forming stable operation space in the oral cavity of a patient, effectively isolating oral treatment operation space and oral non-treatment space and removing waste liquid and saliva generated in the oral cavity of the patient in time; an accessary occlusion assembly is also cpaable of relieving muscle fatigue caused by sustained mouth opening of the patient, the working efficiency is grealty increased, and the diagnosis and treatment risks are reduced.
Owner:郭婷 +2
Combined type intraoral soft tissue ultrasound probe
PendingCN110141275AAccurate checkAvoid wear and tearUltrasonic/sonic/infrasonic diagnosticsSurgeryBuccal mucosaCross infection
The present invention provides a combined type intraoral soft tissue ultrasound probe. The combined type intraoral soft tissue ultrasound probe comprises a probe, an outside of a detection end of theprobe is sleevedly connected with a coating membrane containing a coupling agent, an outside of the coating membrane is sleevedly connected with a detachable coating membrane fixing member, the coating membrane fixing member is hollow, one end of the coating membrane fixing member is provided with a first opening for inserting the probe sleevedly connected with the coating membrane, and a bottom part of the coating membrane fixing member is provided with a second opening exposing the coating membrane. The provided combined type intraoral soft tissue ultrasound probe can be close to intraoral soft tissues such as tongue back, tongue edges, buccal mucosa and lip mucosa, facilitates examination of lesion conditions of the intraoral soft tissues, does not need frequent rubbing and disinfecting, avoids wear, reduces a risk of cross-infection for patients, is provided with an artificially manufactured acoustic window with a fixed thickness, avoids manual adjustment of thickness of the acoustic window during an examination process, increases clarity degrees of ultrasound images, and improves examination accuracy of the intraoral soft tissues.
Owner:SHANGHAI NINTH PEOPLES HOSPITAL SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
Soluble fentanyl, derivative buccal membrane preparation thereof and preparing method thereof
ActiveCN104940173AImprove bioavailabilityRapid pain reliefOrganic active ingredientsNervous disorderBlood concentrationBuccal mucosa
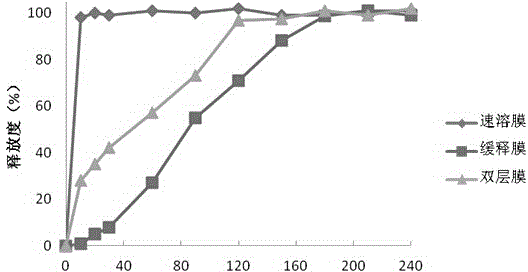
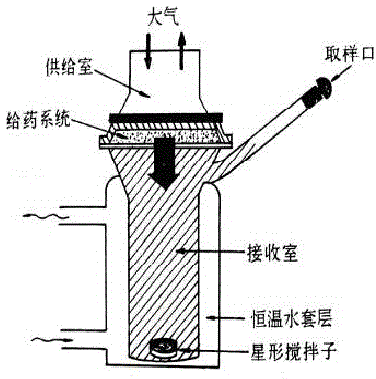
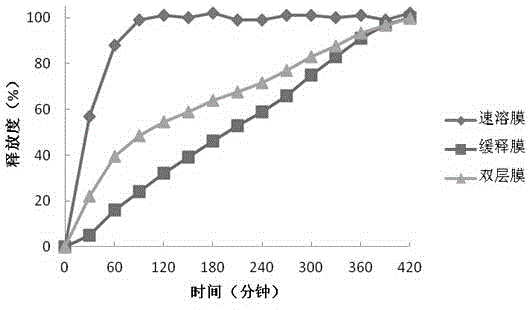
The invention discloses soluble fentanyl, a derivative buccal membrane preparation thereof and a preparing method thereof. The soluble fentanyl and the derivative buccal membrane preparation thereof can be instant fentanyl and a derivative buccal membrane preparation thereof, can be soluble slow-release fentanyl and a derivative buccal membrane preparation thereof and also can be instant and soluble slow-release fentanyl and a derivative buccal double-layer-membrane preparation. An instant membrane can be dissolved quickly at a buccal mucosa in one minute at the oral cavity, the fentanyl and a derivative thereof are released, and the clinical effect of efficient analgesia is achieved. A slow-release membrane slowly releases medicine within four hours in the oral cavity, and effective blood concentration is maintained for a long time. An instant-slow-release double-layer membrane has a quick effect, the medicine effect is durable, and continuous analgesia can be achieved.
Owner:LP PHARM (XIAMEN) CO LTD
Oral cancer detection marker and oral cancer detection kit
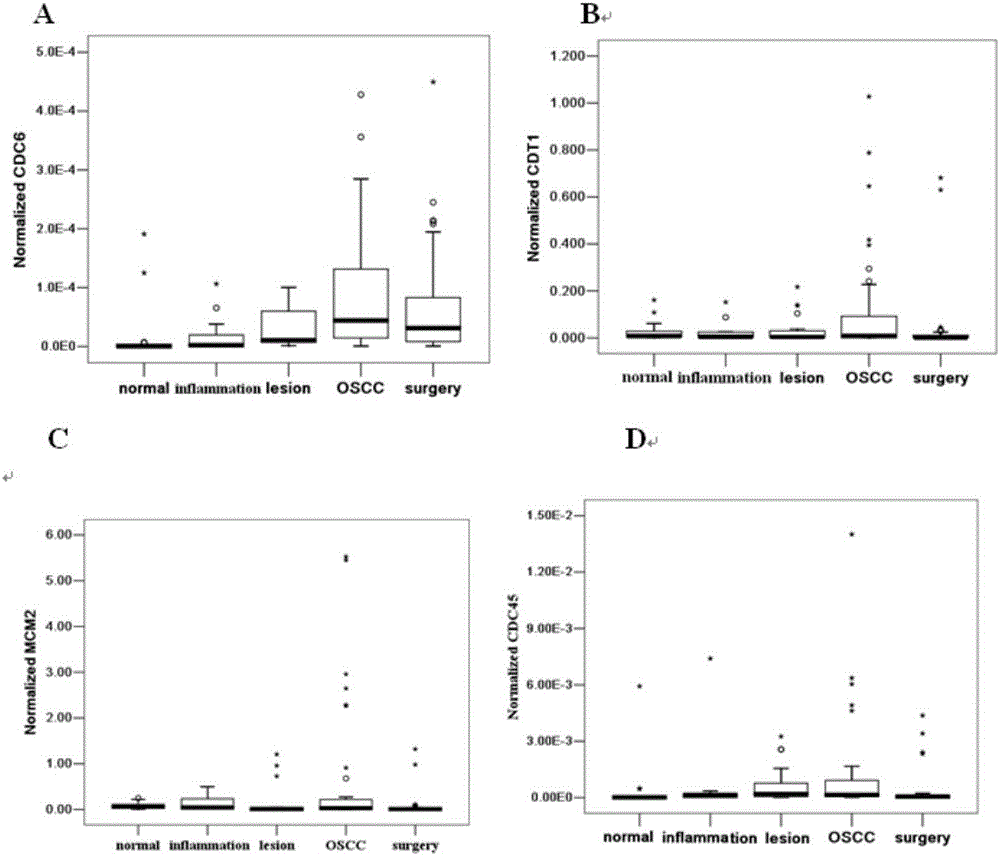
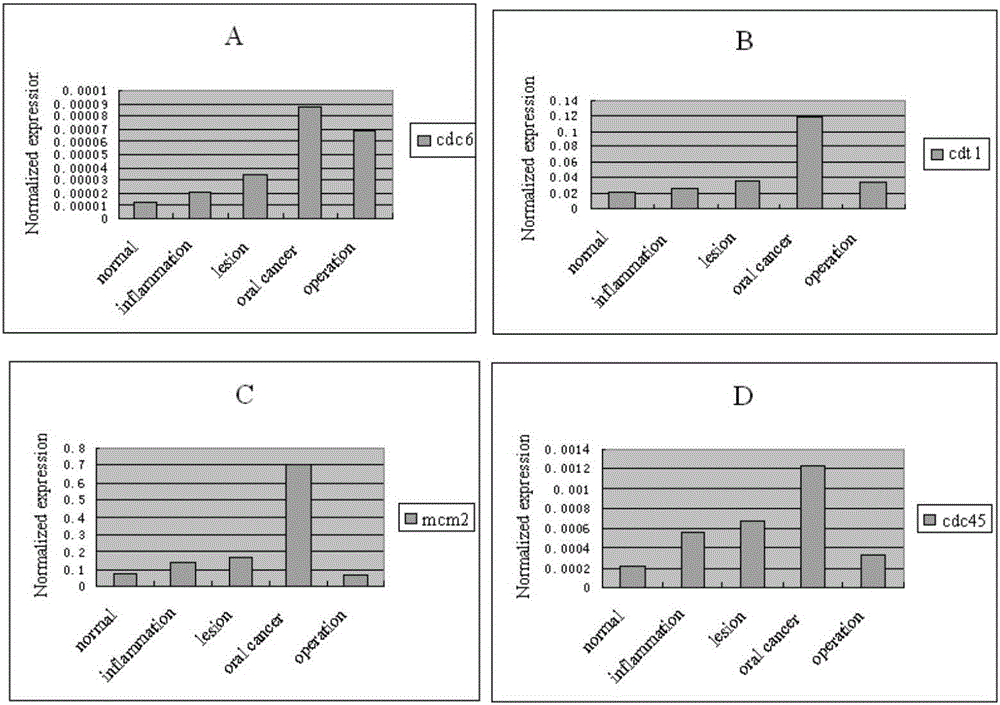
The invention belongs to the technical field of biology and particularly discloses an oral cancer detection marker and an oral cancer detection kit. The levels of mRNA of CDC6, CDT1, MCM2 and CDC45 in buccal mucosal cells of a normal person, a person with oral inflammation, a person with precancerous lesions, a person with oral cancer and a person at the postoperation period are quantitatively detected, the detection shows that CDC6, CDT1, MCM2 and CDC45 are ideal tumor markers for the screening of the oral cavity in the early stage, and the normal buccal mucosal cells and the canceration exfoliated cells are effectively distinguished. The oral cancer detection marker and the oral cancer detection kit are of great significance for the exploration of the mechanism of the content change of the tumor marker in the buccal mucosal cells and the clinical value of prognosis judgement.
Owner:SHENZHEN UNIV
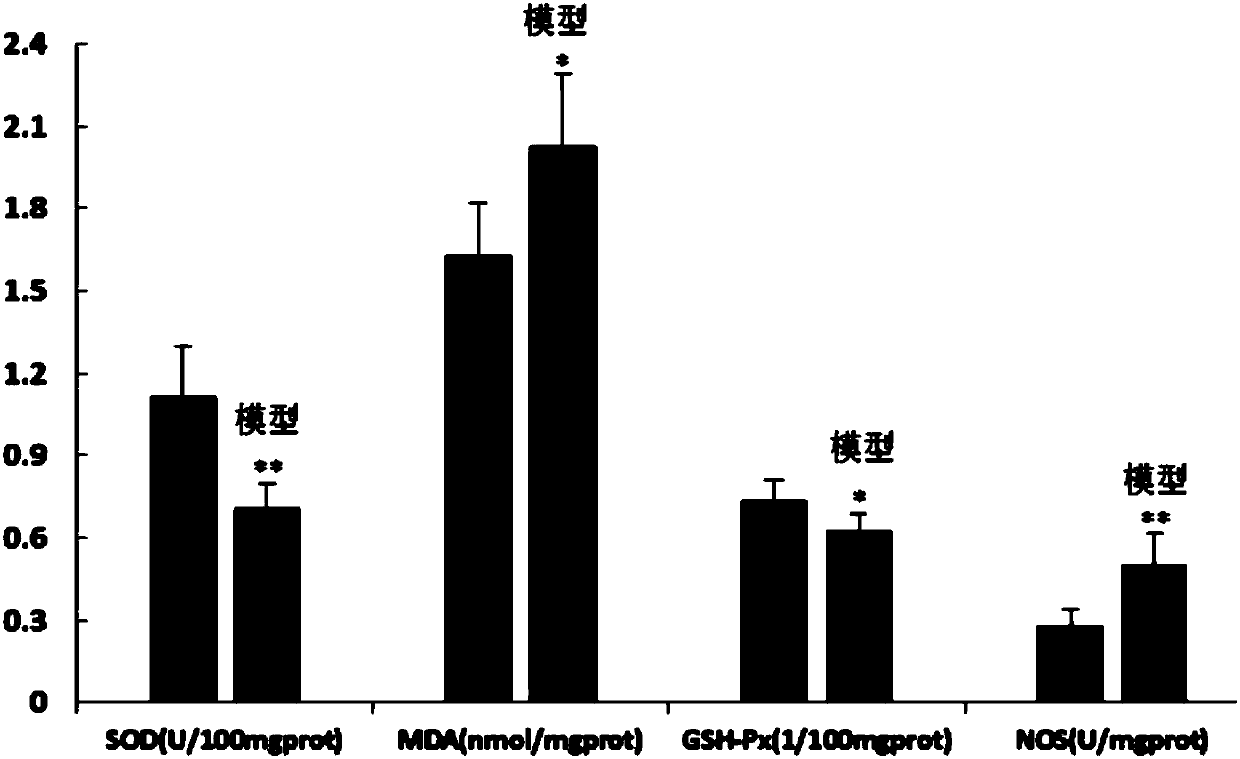
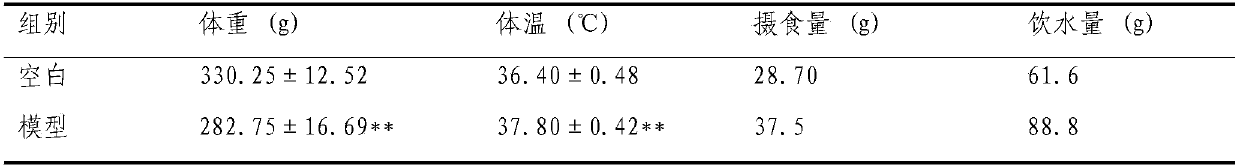
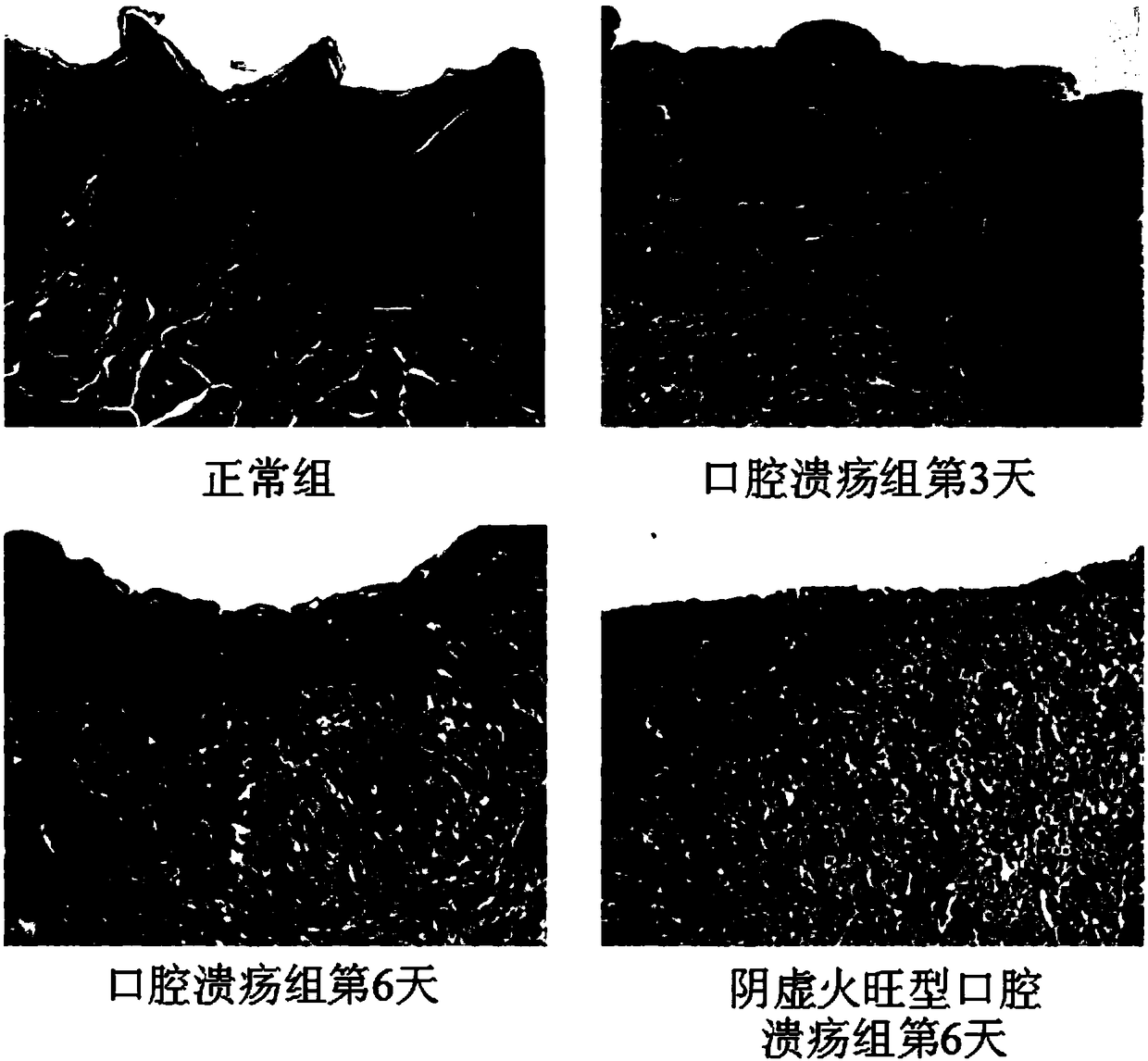
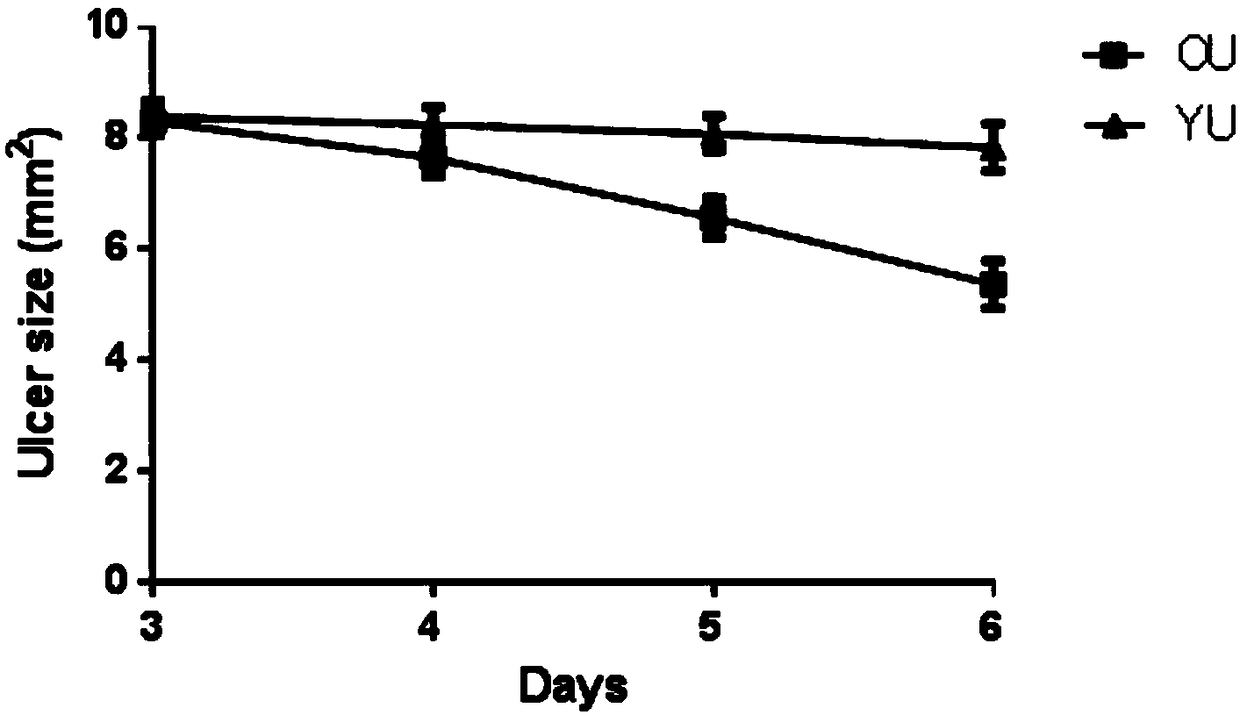
Preparation method of animal model with oral ulcer caused by fire excess from yin deficiency
PendingCN107625760AAvoid damageIn line with clinical practiceHydroxy compound active ingredientsIn-vivo testing preparationsOral ulcersYin deficiency
The invention discloses a preparation method of an animal model with oral ulcer caused by fire excess from yin deficiency. The method comprises selecting healthy rats, carrying out subcutaneous injection on the rats with a triiodothyronine solution according to the dosage of 5-200 micrograms per kilogram for 14-21 days, carrying out impregnation on a cotton ball with phenol, and firing the rat lateral buccal mucosa to form a white wound so that a desired animal model can be obtained after 24-48h. Compared with the prior art, the method is used for preparation of an animal model with oral ulcercaused by fire excess from yin deficiency. The model can induce the oral ulcer based on the fire excess from yin deficiency according to the traditional Chinese medicine differentiation of symptoms and signs, well satisfies clinical practice, has high feasibility and high applicability and has a great application value in heat and fire removal, tissue damage reduction and ulcer healing promotion.
Owner:SUN YAT SEN UNIV
An adjustable orthodontic mouthpiece
InactiveCN103919621BImprove adhesionAdjust the support strengthOthrodonticsSomatoscopeBuccal mucosaOrthodontics
The invention discloses an adjustable orthodontic mouth gag, which comprises an oral cavity front support, molar region support fins and a molar region support fin regulating spring, wherein the oral cavity front support comprises a connecting rod and two auricles, the connecting rod is of an arc structure, and the sides of the two auricles are connected by the connecting rod to form a whole; each auricle consists of an auricle large wing and an auricle small wing, and the auricle large wing and the auricle small wing are laminated up and down; one molar region support fin is arranged outside each auricle large wing, and the molar region support fin is of a semielliptic laminated structure; two ends of the molar region support fin are respectively connected to the outer surface of the auricle large wing by the molar region support fin regulating spring. The adjustable orthodontic mouth gag has the characteristics that the molar region support fins are arranged on the auricles by the molar region support fin regulating spring and used for strutting the molar region buccal mucosa, so that the adhesion of a cheek teeth region orthodontic accessory is facilitated; the supporting force degree on the molar region buccal mucosa can be adjusted, and the discomfort of the mouth gag in operation is reduced when the operation simpleness is achieved.
Owner:THE SECOND AFFILIATED HOSPITAL OF CHONGQING MEDICAL UNIV
Method for constructing yin-deficiency fire-excess type oral ulcer animal model based on sleep deprivation
InactiveCN109105336ALong durationHydroxy compound active ingredientsSurgical veterinaryEtiologyOral ulcers
The invention relates to a method for constructing a yin-deficiency fire-excess type oral ulcer animal model based on sleep deprivation. The method comprises the following steps: buccal mucosa of a rat is burnt by using phenol, so that white damage is caused, and an oral ulcer animal model is obtained after 24-48 hours; the animal model is placed in a multi-platform water-environment device for 2-7 days, wherein the multi-platform water-environment device is composed of a mouse box and a plurality of platforms, and the plurality of platforms are arranged in the mouse box and have diameters of4-8 cm and distance of 10-20 cm are arranged in the platform; and the place among the platforms is filled with water, and the height of the platform is about 0.1-2 cm higher than the water surface. The invention overcomes the defects that in the prior art, the evaluation index is single, the model duration time is short, the etiology is not matched with clinic, and the like.
Owner:SUN YAT SEN UNIV
Method for the delivery of a biologically active agent
A method of manufacturing a stable nanosuspension for delivery of a biologically active agent into the bloodstream of a subject is disclosed. A microfluidizable mixture is initially formed and processed via a microfluidization process to form the stable nanosuspension, which may be administered via the buccal mucosa or other suitable routes of administration. This product demonstrates increased bioavailability, enhanced period of onset, and enhanced stability for a controlled-release product.
Owner:HEALTH PLUS INT
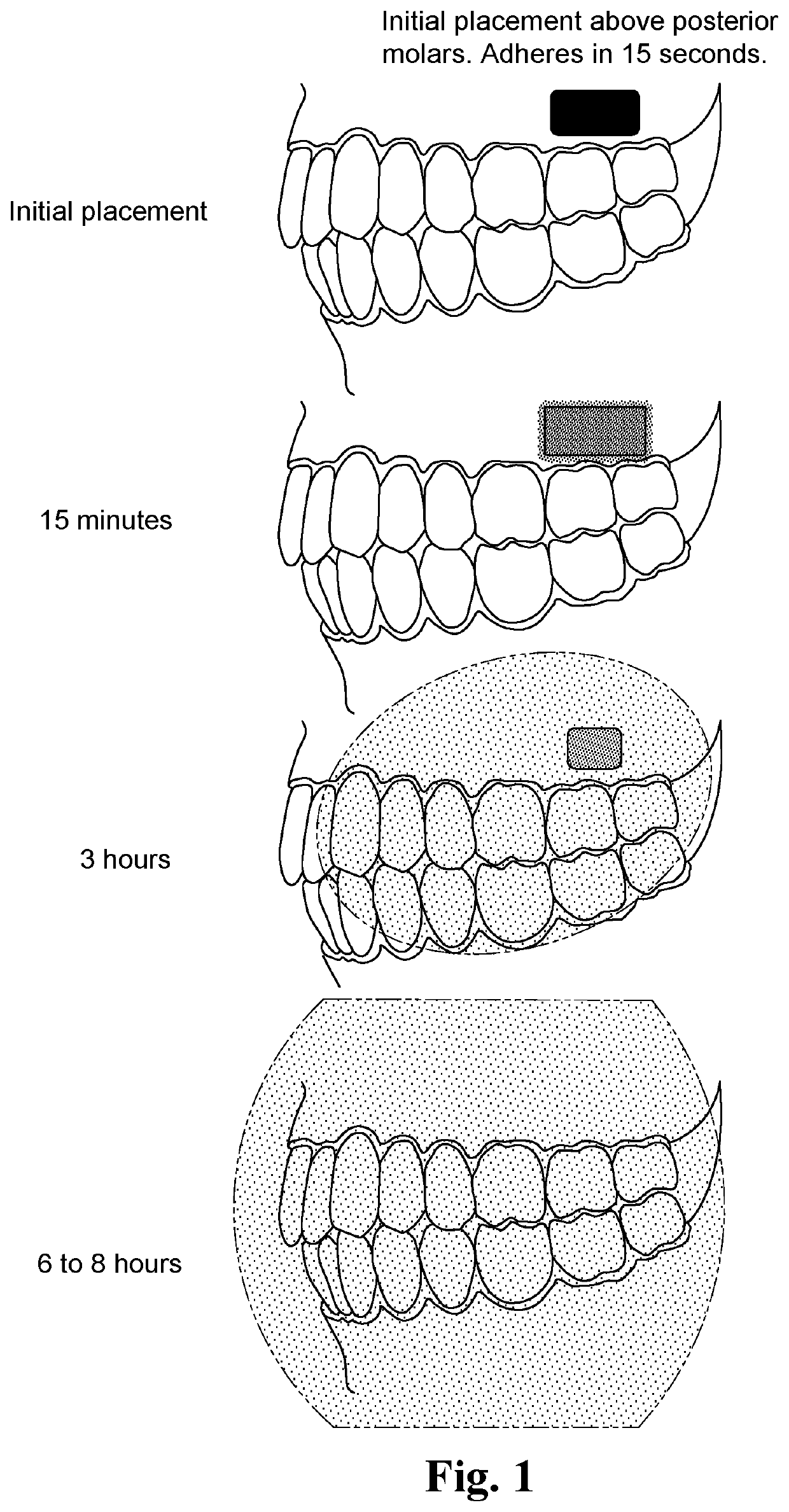
Adherent oral pharmabiotic delivery strip
ActiveUS11058634B2Large and malleable surface areaMaximize acceptancePeptide/protein ingredientsHydroxy compound active ingredientsPharmaceutical drugBuccal mucosa
An oral pharmabiotic system is disclosed for improving oral, dental, and systemic health by repopulating and reshaping the flora within a patient's oral environment in a manner that overcomes the deficiencies of prior oral probiotic products. By formulating the pharmabiotic system as a strip for adhesive placement within a patients' oral cavity, preferably against the buccal mucosa, alveolar mucosa, oral labial mucosa, or a dental appliance, and configuring the parameters of the strip such that neither disadhesion nor complete dissolution occurs for at least a period of at least three hours during daytime use and at least six hours during nighttime use, the probiotic payload contained within may remain in the oral cavity for a sufficient length of time required for the probiotics to activate, replicate, and displace existing harmful oral pathobiotics.
Owner:EDWARDS STEVEN J
Intra-Oral Appliance for Field Isolation and Moisture Control
ActiveUS20190167383A1Increase the length dimensionIncrease effective lengthDental splintsSaliva removersBuccal mucosaCheek
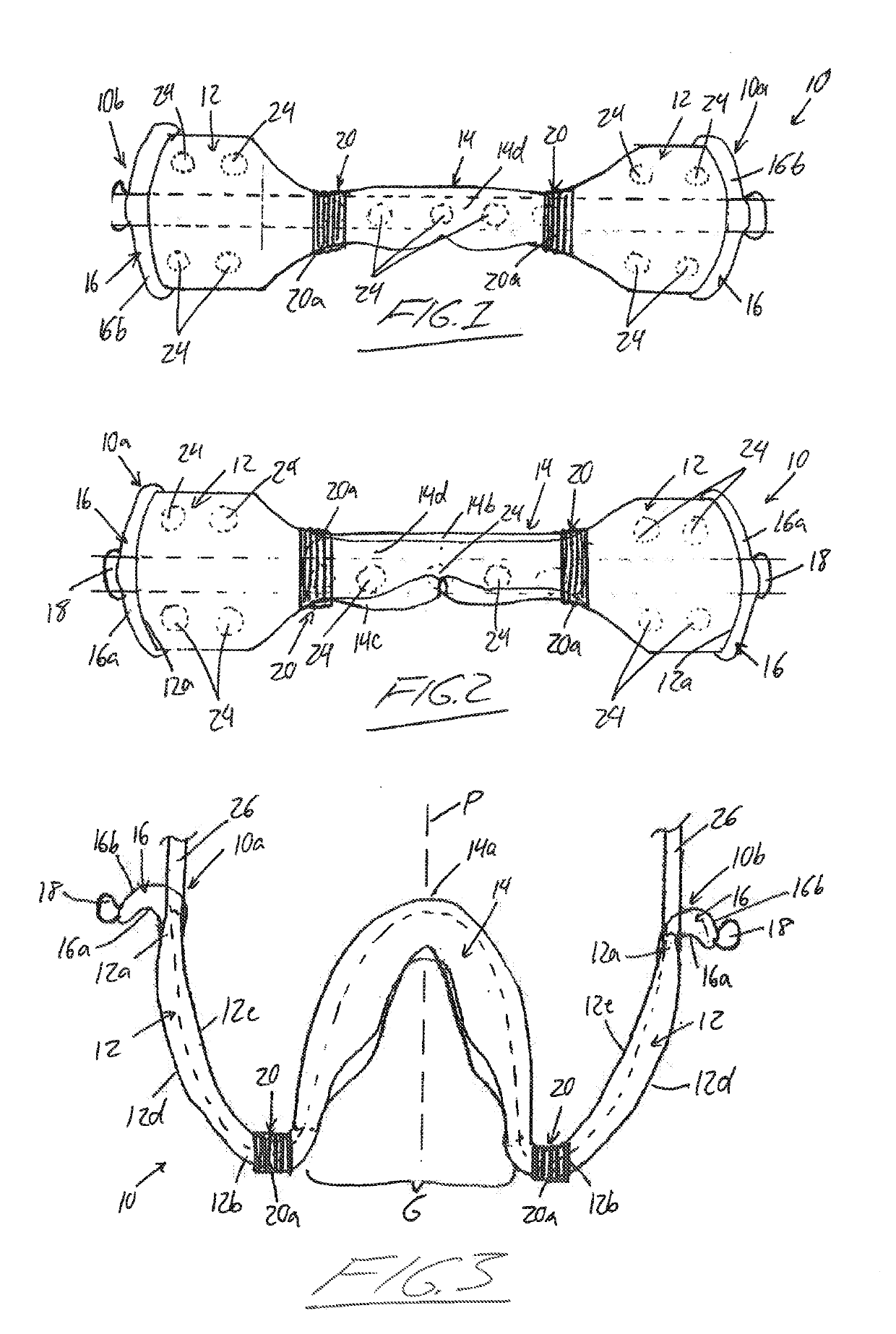
An intra-oral simultaneous bilateral inter arch isolation appliance features a generally W-shaped body having first and second cheek retractor wings, and first and second transitions respectively joining the cheek retractor wings to a tongue crib of generally U-shaped configuration with an open posterior end. In a patient worn position, each cheek retractor wing runs buccally along a respective half of a patient's dental arches to retract and isolate buccal mucosa, while the transitions curve posteriorly around the rear molars between the maxillary tuberosity and retromolar pad to carry the tongue crib inside the dental arches, where it shields and isolates the tongue. Commissure cradles at anterior ends of the cheek retractor wings hook around the patient's oral commissures at terminal ends of the appliance. Inner passageways within the body accommodate fluid extraction and / or light transmission through the appliance.
Owner:CHANA RANDEEP +1
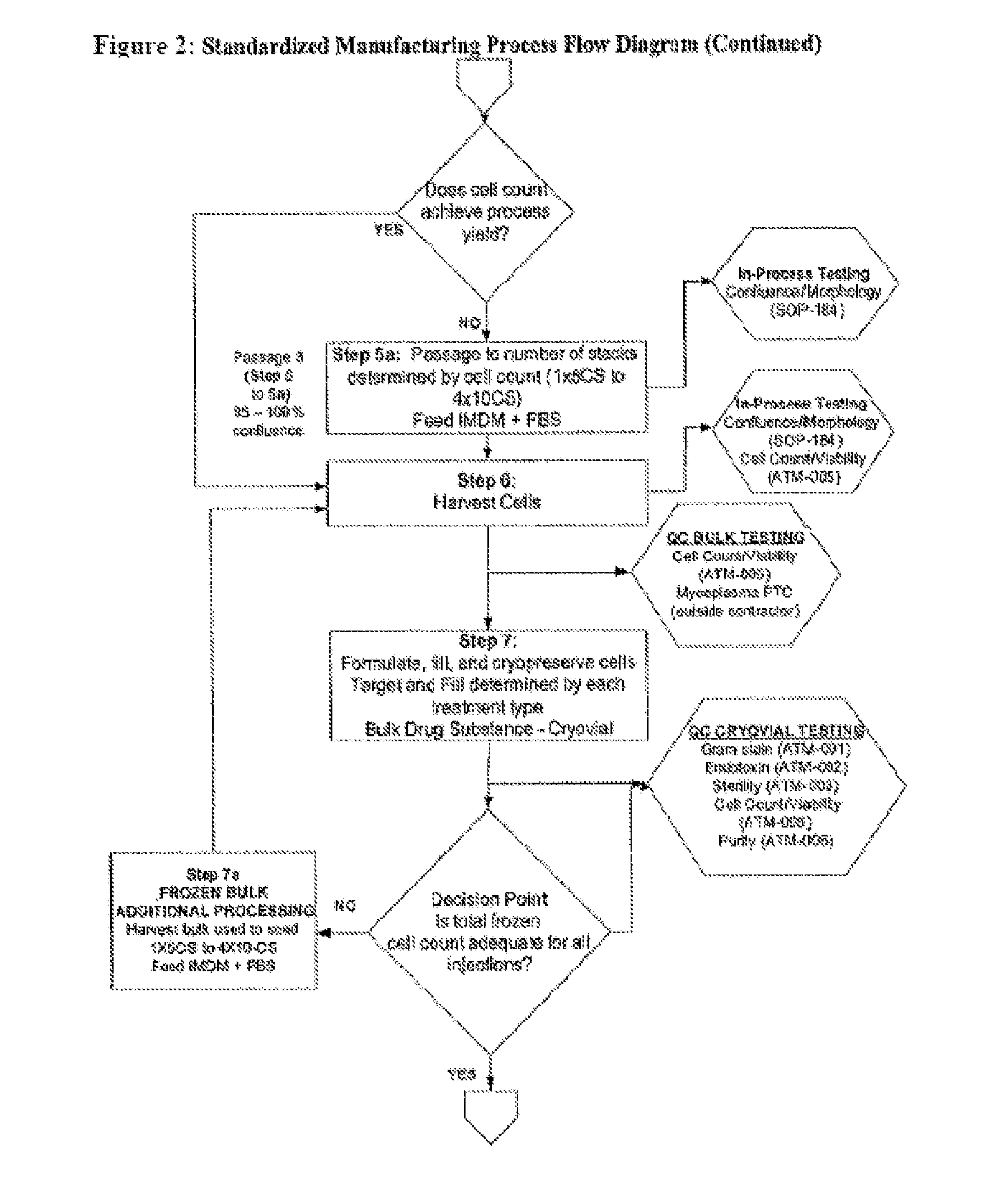
Treatment of Vocal Cords with Autologous Dermal Fibroblast Formulation
Dosage units consist of an autologous cell therapy product composed of fibroblasts grown for each individual to be treated for augmentation or regeneration of vocal cords. The suspension of autologous fibroblasts, grown from a biopsy of each individual's own buccal mucosa or skin using current good manufacturing practices (CGMP) and standard tissue culture procedures, is supplied in vials containing cryopreserved fibroblasts or precursors thereof, having a purity of at least 98% fibroblasts and a viability of at least 85%, for administration of from one to six mL, preferably two mL administered three times approximately three to six weeks apart, of cells at a concentration of from 1.0-2.0×107 cells / mL.
Owner:CASTLE CREEK BIOSCIENCES LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com