Olanzapine medicine absorbed through oral mucosa
An oral mucosa and olanzapine technology, which is applied in the field of olanzapine drugs, can solve the problems of difficulty in quickly controlling the symptoms of schizophrenia in the acute phase, patients with mental illness not cooperating with treatment, and difficulty in guaranteeing treatment effects, so as to avoid the first pass of the liver. effect, improved bioavailability, fast-acting effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
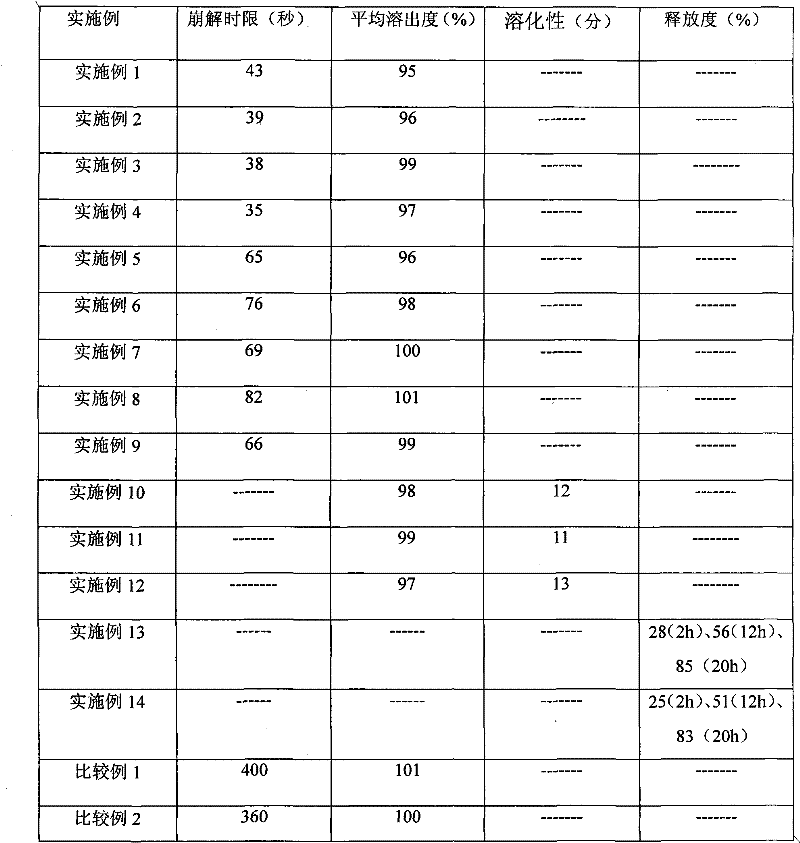
Examples
Embodiment 1
[0023] Embodiment 1 (sublingual tablet)
[0024] composition:
[0025] Olanzapine 2.5g
[0026] Microcrystalline Cellulose 30g
[0027] Mannitol 25g
[0028] Sodium carboxymethyl starch 3g
[0029] Magnesium stearate 0.5g
[0030]
[0031] Made into 1000 pieces
[0032] Preparation method: Olanzapine is micronized with a jet mill to a particle size of D 90 ≤50μm, weighed according to the prescription, mixed evenly with the prescription amount of auxiliary ingredients such as microcrystalline cellulose and mannitol, directly compressed into tablets, and the hardness is controlled at 3-5kg, that is to say.
Embodiment 2
[0033] Embodiment 2 (sublingual tablet)
[0034] composition:
[0035] Olanzapine 5g
[0036] Starch 20g
[0037] Microcrystalline Cellulose 30g
[0038] 10g pregelatinized starch
[0039] Crospovidone 4g (half inside and outside)
[0041]
[0042] Made into 1000 pieces
[0043] Preparation method: Olanzapine is micronized with a jet mill to a particle size of D 90 ≤30μm, mix the prescribed amount of olanzapine with auxiliary ingredients such as starch and microcrystalline cellulose, granulate with pure water as wetting agent, dry, granulate, add crospovidone and talc powder, mix Evenly, press into tablets, the hardness is controlled at 3-5kg, that is to say.
Embodiment 3
[0044] Embodiment 3 (sublingual tablet)
[0045] composition:
[0046] Olanzapine 3.5g
[0047] Lactose 30g
[0048] 40g pregelatinized starch
[0049] Croscarmellose Sodium 6g
[0050] Stevioside 5g
[0051] Peppermint Oil 0.1g
[0053]
[0054]Made into 1000 pieces
[0055] Preparation method: use a ball mill to pulverize olanzapine to a particle size of D 90 ≤80μm, after uniformly mixing olanzapine, lactose, pregelatinized starch and other auxiliary ingredients, directly compressing tablets, the hardness is controlled at 2-4kg, that is.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com