Sunitinib malate liposome and preparation method thereof
A technology of sunitinib malate lipid and liposome preparation, which is applied in the field of medicine to achieve the effect of no significant change
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0011] Hydrogenated soybean lecithin (HSPC) 3g
[0012] Cholesterol (CH) 1g
[0013] mPEG 2000 -DSPE 0.75g
[0014] Weigh each lipid compound in the prescription, stir and dissolve with an appropriate amount of absolute ethanol in a 65°C water bath, evaporate the ethanol, and add a certain concentration of ammonium sulfate ((NH 4 ) 2 SO 4 ) solution, stirred in a water bath at 65°C for 20 min to obtain the primary blank liposome. Sonicate the primary product probe for 8 min (200 w×2 min, 400 w×6 min), and pass through 0.8, 0.45, 0.22 μm microporous membranes in turn to obtain a blank liposome suspension, and the final phospholipid mass concentration 50 mg·mL -1 .
[0015] 2 General scheme 2
[0016] Blank liposome prescription
[0017] Hydrogenated soybean lecithin (HSPC) 3g
[0018] Cholesterol (CH) 1g
[0019] mPEG 2000 -DSPE 0.75g
[0020] Weigh each lipid compound in the prescription, stir and dissolve with an appropriate amount of absolute ethanol in a 70°C w...
Embodiment 2
[0031] Take an appropriate amount of gradient liposome and SU solution (5.0 mg·mL -1 ) and incubate at a certain temperature for a certain period of time to obtain SU-L.
[0032] Determination of SU-L Encapsulation Efficiency
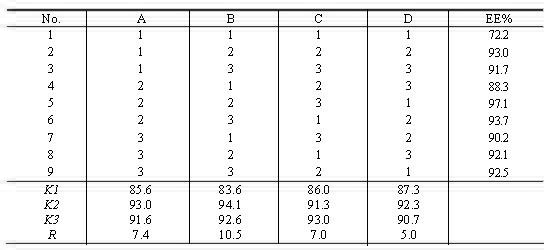
[0033] The encapsulation efficiency of SU-L was determined by the cation exchange resin method, and the specific operation was as follows: Precisely pipette 0.1 mL of SU-L into a 10.0 mL volumetric flask, add 1.6 mL of distilled water, and make a volume fraction of 90% isopropanol solution (containing 0.75 mol·L -1 HCl) demulsification and constant volume, shake well, measure absorbance A at 430 nm wavelength 0 (total drug absorption). Another precision pipette 0.1 mL SU-L, placed in the center of the top surface of the cation exchange resin column, 2000 r min -1 Centrifuge for 4 min, then add 400 μL of distilled water to the top of the column, 2000 r min -1 Centrifuge for 4 min, repeat 4 times, and combine eluents. The eluate was transferred to a...
Embodiment 3
[0062] Cholesterol (CH) 1g
[0063] Macrogol 1000 Cholesterol Hemisuccinate (mPEG1000-CHEMS) 0.5g
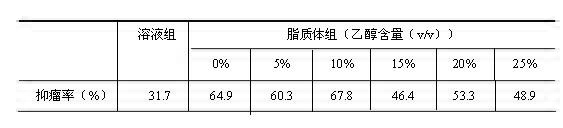
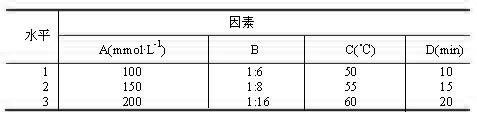
[0064] Weigh the prescription quantities of DSPC, CH, mPEG1000-CHEMS, and use 2.5%, 5.0%, 10%, 15%, 20%, 25%, 30% and 40% (volume ratio of ethanol to hydration medium) of absolute ethanol respectively Dissolve the membrane material, add 150 mmol·L -1 of (NH 4 ) 2 SO 4 solution, stirred in a water bath at 65°C for 20 min to obtain the primary blank liposome, and the final phospholipid mass concentration was 50 mg·mL -1 . Sonicate the primary product probe for 8 min (200 w×2 min, 400 w×6 min), then pass through the microporous membranes of 0.8, 0.45, and 0.22 μm in sequence to obtain a blank liposome suspension, which was measured by laser particle size The particle size of each liposome was measured by an instrument, and the results showed that when the residual ethanol content did not exceed 10% (v / v), the liposome particle size did not change significantly between 108.2 a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com