Preparation method of sunitinib malate crystal form (I)
A technology of sunitinib malate and its crystal form, which is applied in the field of preparation of crystal form I, and can solve problems such as high vacuum requirements, unsuitability for industrial production applications, and low solvent residue limit
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
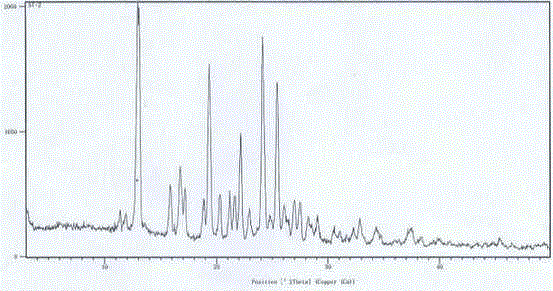
Embodiment 1
[0017] Embodiment 1N-[2-(diethylamino) ethyl]-5-[( Z )-(5-fluoro-1,2-dihydro-2-oxo-indol-3-enyl)methyl]-2,4-dimethyl-1 H -Pyrrole-3-amide ( L )- Preparation of malate crystal form Ⅰ
[0018] N-[2-(diethylamino) ethyl]-5-[( Z )-(5-fluoro-1,2-dihydro-2-oxo-indol-3-enyl)methyl]-2,4-dimethyl-1 H -Pyrrole-3-amide (75.0g) and methanol (1500ml) were added to the reaction flask, under nitrogen protection, stirred at room temperature for 30 minutes, and L-malic acid (25.0g) was added at one time, the reaction solution dissolved instantly, and then there was a lot of yellow The solid precipitated, and the reaction solution was heated to 50°C and stirred for more than 1 hour, then cooled to room temperature (25°C), concentrated under reduced pressure, and the obtained solid was vacuum-dried at 40°C for more than 4 hours to obtain 100.0 g of an amorphous or slightly crystalline crude product.
[0019] Add 100g of the above crude product and 400ml of dimethyl sulfoxide (DMSO) into the ...
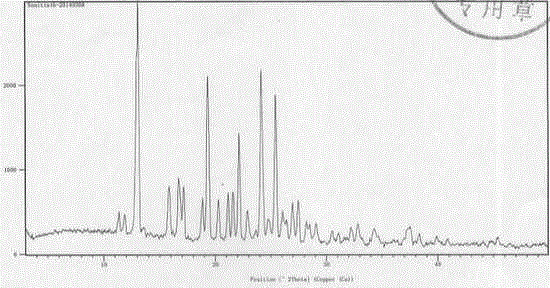
Embodiment 2
[0020] Embodiment 2N-[2-(diethylamino) ethyl]-5-[( Z )-(5-fluoro-1,2-dihydro-2-oxo-indol-3-enyl)methyl]-2,4-dimethyl-1 H -Pyrrole-3-amide ( L )- Preparation of malate crystal form Ⅰ
[0021] N-[2-(diethylamino) ethyl]-5-[( Z )-(5-fluoro-1,2-dihydro-2-oxo-indol-3-enyl)methyl]-2,4-dimethyl-1 H -Pyrrole-3-amide (75.0g) and methanol (1500ml) were added to the reaction flask, under nitrogen protection, stirred at room temperature for 30 minutes, and L-malic acid (25.0g) was added at one time, the reaction solution dissolved instantly, and then there was a lot of yellow The solid precipitated, and the reaction solution was heated to 50°C and stirred for more than 1 hour, then cooled to room temperature (25°C), concentrated under reduced pressure, and the obtained solid was vacuum-dried at 40°C for more than 4 hours to obtain 100.0 g of an amorphous or slightly crystalline crude product.
[0022] Add 100g of the above crude product and DMSO (400ml) into the reaction flask, and st...
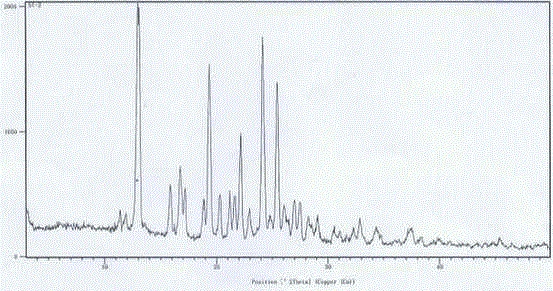
Embodiment 3
[0023] Embodiment 3N-[2-(diethylamino) ethyl]-5-[( Z )-(5-fluoro-1,2-dihydro-2-oxo-indol-3-enyl)methyl]-2,4-dimethyl-1 H -Pyrrole-3-amide ( L )- Preparation of malate crystal form Ⅰ
[0024] N-[2-(diethylamino) ethyl]-5-[( Z )-(5-fluoro-1,2-dihydro-2-oxo-indol-3-enyl)methyl]-2,4-dimethyl-1 H -Pyrrole-3-amide (75.0g) and methanol (1500ml) were added to the reaction flask, under nitrogen protection, stirred at room temperature for 30 minutes, and L-malic acid (25.0g) was added at one time, the reaction solution dissolved instantly, and then there was a lot of yellow The solid precipitated, and the reaction solution was heated to 50°C and stirred for more than 1 hour, then cooled to room temperature (25°C), concentrated under reduced pressure, and the obtained solid was vacuum-dried at 40°C for more than 4 hours to obtain 100.0 g of an amorphous or slightly crystalline crude product.
[0025] Add 100g of the above crude product and DMSO (400ml) into the reaction flask, and st...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com