A kind of preparation method of 1-malate sunitinib e-form isomer
A sunitinib malate and isomer technology, which is applied in the field of preparation of the E-isomer of sunitinib malate, can solve problems such as the E-isomer of sunitinib that is not mentioned , to achieve the effect of high product yield, mild reaction conditions and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0022] The preparation of L-malate sunitinib E-form isomer is obtained by the following technical route:
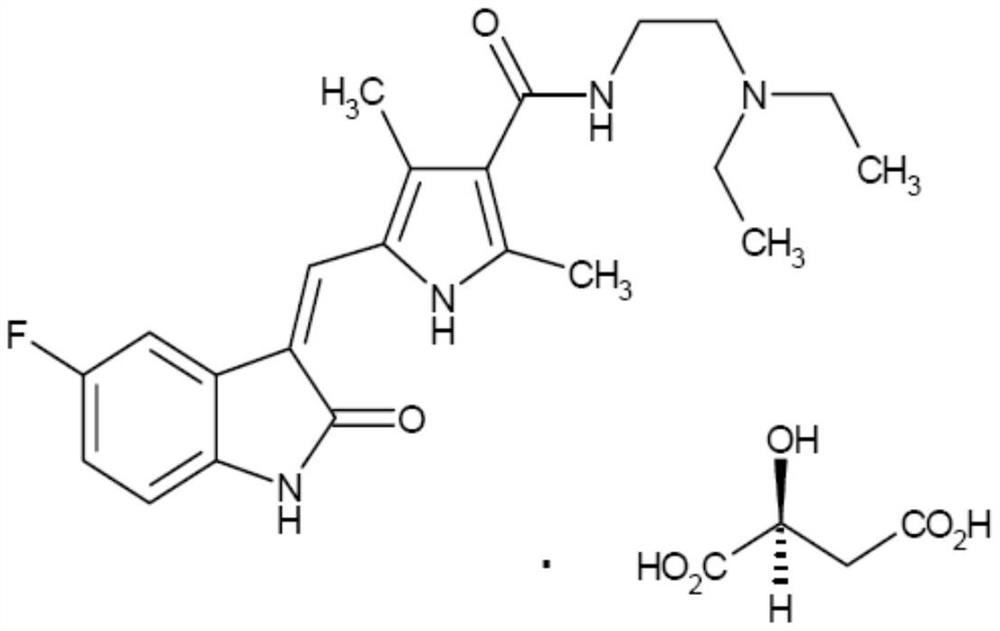
[0023] The raw material drug L-malate sunitinib ((Z)-N-[2-(diethylamino)ethyl]-5-[(5-fluoro-2-oxo-1, 2-Dihydro-3H-indole-3-ylidene)methyl]-2,4-dimethyl-3-carbamoyl-1H-pyrrole L-malate) was synthesized by using existing technology.
Embodiment 1
[0029] Example 1: (E)-N-[2-(diethylamino)ethyl]-5-[(5-fluoro-2-oxo-1,2-dihydro-3H-indole-3 -subunit)methyl]-2,4-dimethyl-3-carbamoyl-1H-pyrrole L-malate preparation
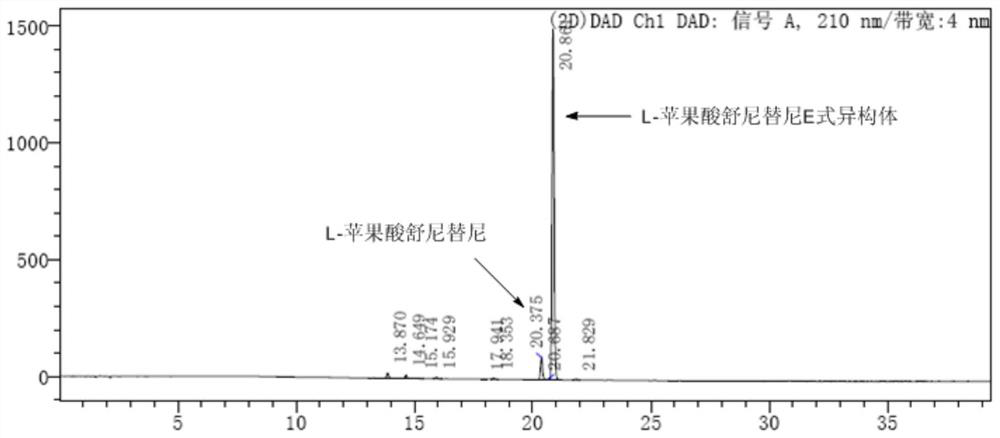
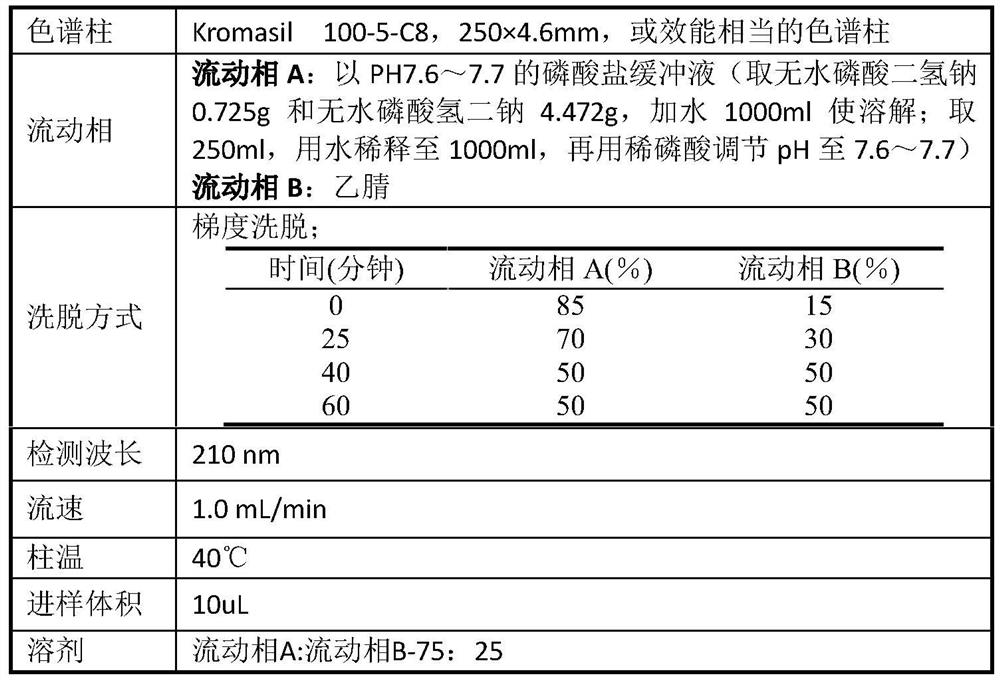
[0030] (Z)-N-[2-(diethylamino)ethyl]-5-[(5-fluoro-2-oxo-1,2-dihydro-3H-indole-3-ylidene ) methyl]-2,4-dimethyl-3-carbamoyl-1H-pyrrole L-malate (0.5 g, 0.89 mmol) was dissolved in 300 mL of water, 5.3 mg of anthracene was added, and after mixing evenly, it was placed in In the photoreactor, turn on a 500W medium pressure mercury lamp, control the temperature at 0-5°C and react for 16-18h, stop the reaction when the liquid phase monitoring conversion rate is greater than 90%, filter, and store the filtrate in a freeze dryer at -50°C in the dark. Lyophilized at ~-60℃ for 24h to obtain (E)-N-[2-(diethylamino)ethyl]-5-[(5-fluoro-2-oxo-1,2-dihydro-3H -Indole-3-ylidene)methyl]-2,4-dimethyl-3-carbamoyl-1H-pyrrole L-malate 0.32g, yield 64%, HPLC purity 91% (as figure 1 ).
Embodiment 2
[0031] Example 2: (E)-N-[2-(diethylamino)ethyl]-5-[(5-fluoro-2-oxo-1,2-dihydro-3H-indole-3 -subunit)methyl]-2,4-dimethyl-3-carbamoyl-1H-pyrrole L-malate preparation
[0032] (Z)-N-[2-(diethylamino)ethyl]-5-[(5-fluoro-2-oxo-1,2-dihydro-3H-indole-3-ylidene ) methyl]-2,4-dimethyl-3-carbamoyl-1H-pyrrole L-malate (0.5 g, 0.89 mmol) was dissolved in 200 mL of ethanol, 5 mg of anthracene was added, and after mixing uniformly, set In the light-incident reactor, turn on a 500W medium-pressure mercury lamp, control the temperature at 0-5°C and react for 16-18h, stop the reaction when the liquid phase monitoring conversion rate is greater than 90%, and store the filtrate in a freeze dryer at -50°C~ -60°C lyophilization for 24h to obtain (E)-N-[2-(diethylamino)ethyl]-5-[(5-fluoro-2-oxo-1,2-dihydro-3H- Indole-3-ylidene)methyl]-2,4-dimethyl-3-carbamoyl-1H-pyrrole L-malate 0.22 g, yield 44%, HPLC purity 90%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com