Stable butylphthalide sodium chloride injection as well as preparation method and application thereof
A technology of butylphthalide sodium chloride and phthalide sodium chloride is applied in the field of pharmaceutical preparations, which can solve the problems that cannot completely reduce the incompatibility of patients with activated carbon, cannot meet the safety requirements of clinical medication, and the pH value is not well carried out. control and other issues, to achieve the effect of good water solubility, good stability and safety, and low nephrotoxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048]This example discloses the preparation method of biphenylphthalide injection of the niosine chloride of the present invention. The preparation prescription (100 definitions) is:
[0049]
[0050]Preparation method: Return of water, purify water, cooled to 80 ° C after boiling. The sodium sulfur-bust-free cyclodextrin (substituted 6.2 to 6.9, the average substitution degree of 6.4, the molecular weight is 2145.2 g / mol) 80 g, 90 g of sodium chloride, and 8 l 80 ° C, purified water, stir the complete dissolution. 2.5 g of biphenyl phthalide was weighed, and a sulfur dilobea cyclodextride solution was added, and the oil bath was stirred at 80 ° C for 90 minutes to completely dissolve. After cooling to room temperature, add 0.2 M hydrochloric acid solution pH to 4.5, supplement purified water to 10L. The vacuum pump was filtered through a circulating hydraulic pump. The mixed solution is filled with 100 ml / branch. After filling, at 121 ° C, high temperature was sterilized for 15 minu...
Embodiment 2
[0052]This example discloses the amount of sodium sodium sulfur-dioximale cyclodextrin to the solubilization ability of biphenylphthalide, specifically:
[0053]The sodium busenphthalide injection in accordance with Example 1 was prepared, and the difference was only different from sodium sodium sulfurine cyclodextrin. Then, the traits of each butylphthalonate injection of each butyl phthal chloride and a biphenylphtarid content in the injection. The result is shown in Table 1:
[0054]Table 1, solubilizing capacity of sodium sodium sulfur dioxide cyclodextrin solution
[0055]
[0056]
[0057]It can be seen from the above table: sulfurilataltite cyclodextride: biphenitis ratio is greater than 2.8 (numbers 1 and 4), thickebium phthalide can be completely dissolved; when molar ratio is reduced to 1.5, the remaining A small amount of butyl phthalide is reduced by molar ratio to 0.44, the remaining large quantum butyl phthalide droplets, indicating that the quantum donut is 0.44 mol of butylphthalid...
Embodiment 3
[0064]This example investigates the effect of pH value on the stability of sodium biphenyl chloride injection.
[0065]According to the method of Example 1, the busephthalide injection is prepared, and the difference is only the pH value of the adjusted biphenylphthalide injection, the specific pH value of the sodium chloride injection of the prepared Table 3 shows. The impurity content in the injection of the filling and sterilization was investigated, and the results of the examination were shown in Table 3.
[0066]Table 3, different pH affects the stability of the embodiment
[0067]
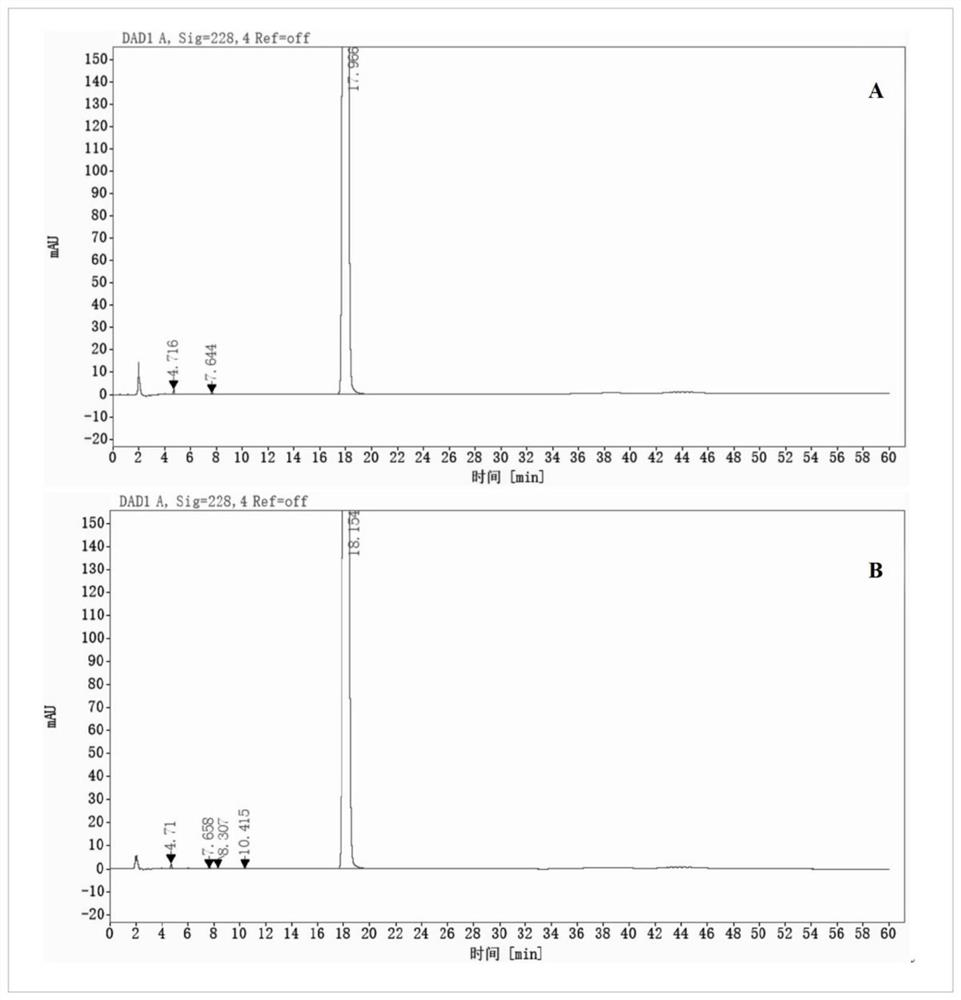
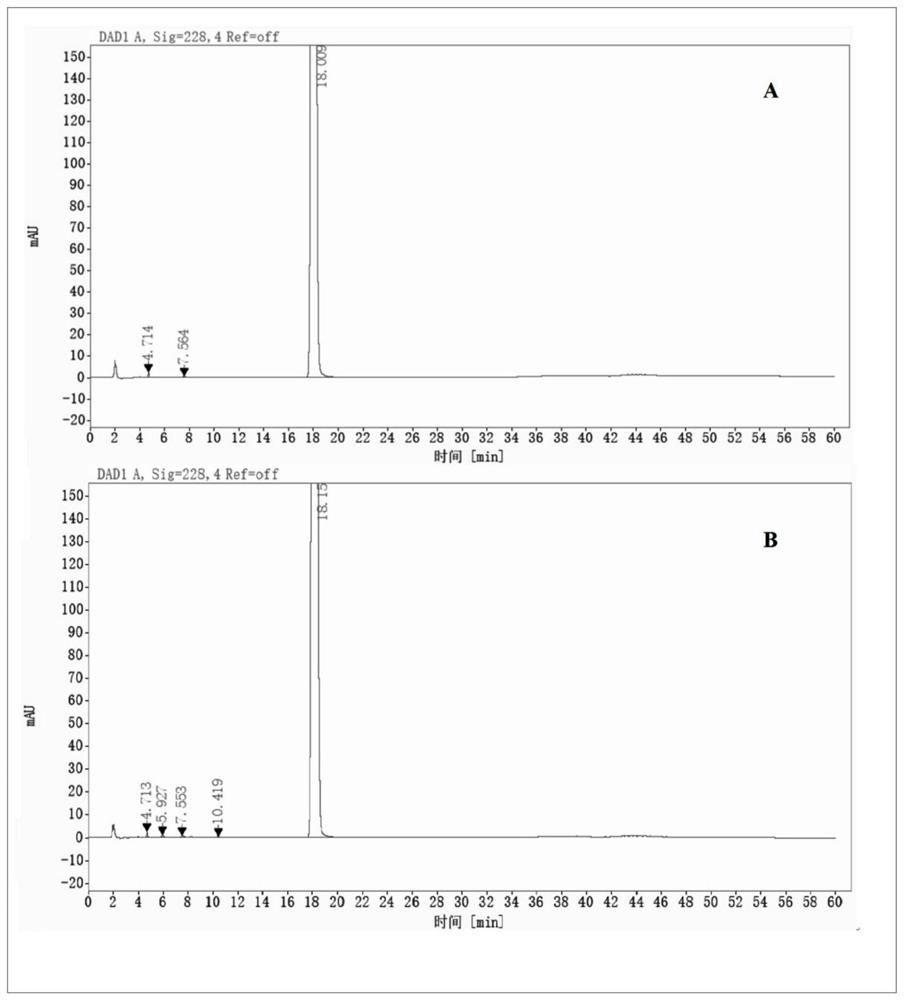
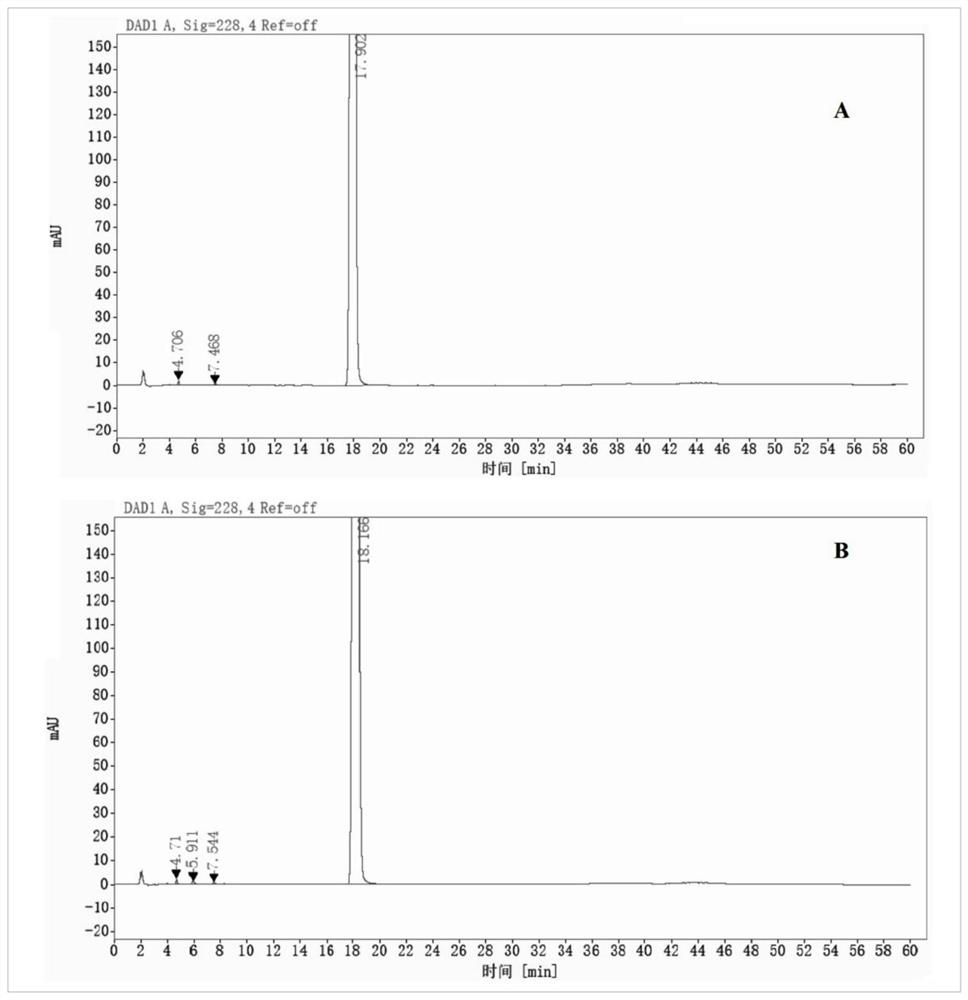
[0068]It can be seen from Table 3, but the phenylphaxa-sodium chloride injection of the pH is 4.0 to 5.0 is sterilized at 121 ° C, 15 min, and there is no significant change in impurity content, and the maximum unknown single proposal is derived from the feedstock, indicating buthen phthal chloride. The sodium sodium injection is good in the pH 4.0 ~ 5.0.Figure 1-3 Indicated. .
[0069]AppendFigure 4-6As shown, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of substitution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com